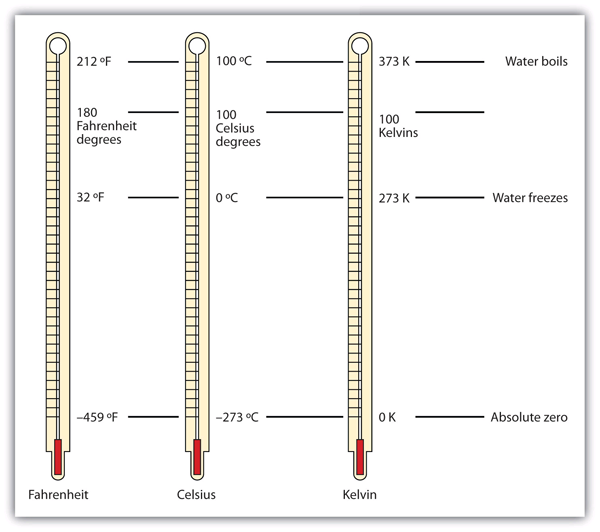
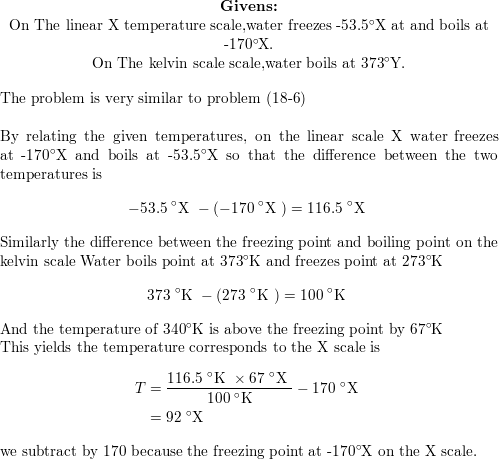
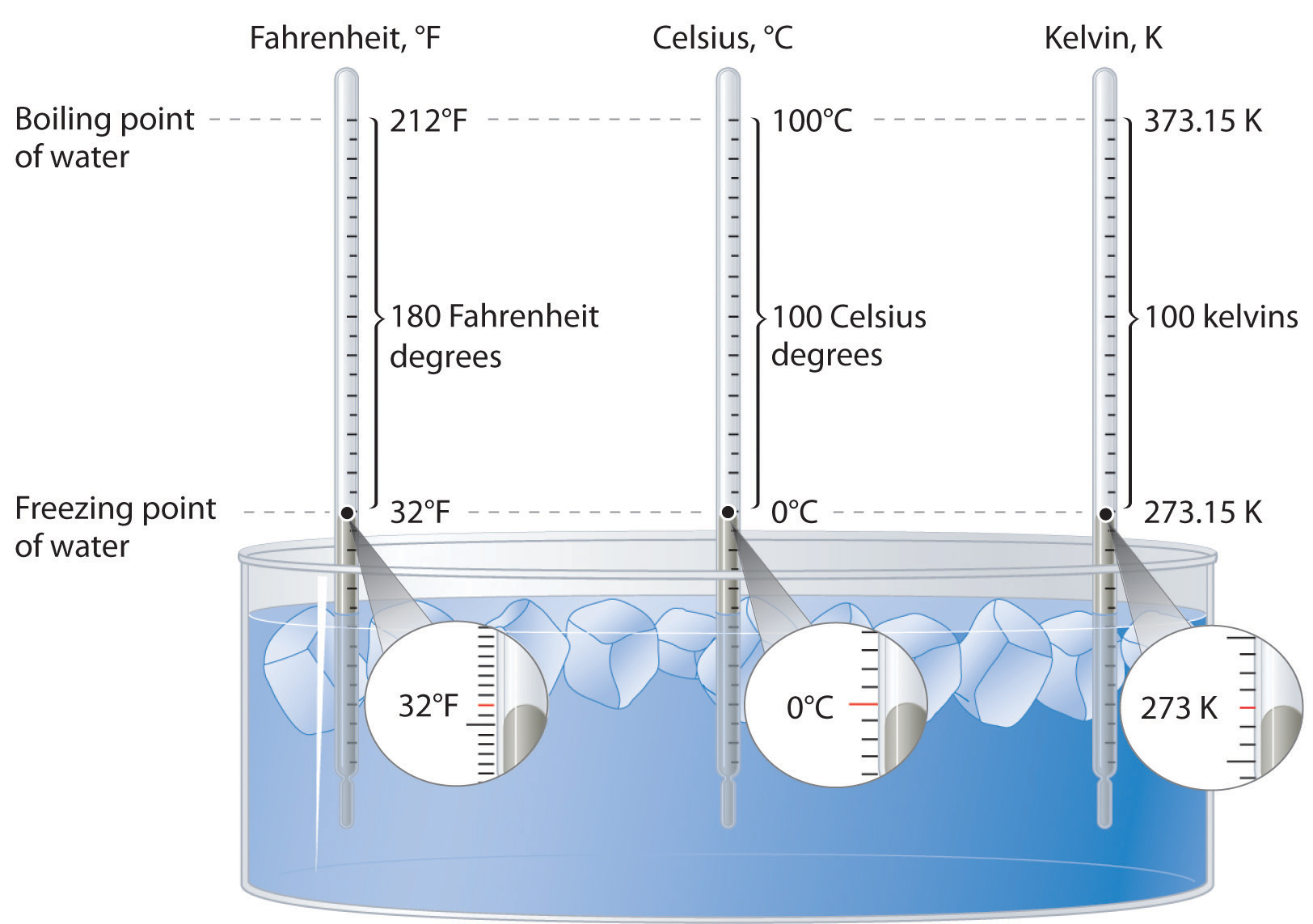
The normal boiling point of water is 373 k. vapour of waterr at temperature T is 19 mm hg. If en... - YouTube
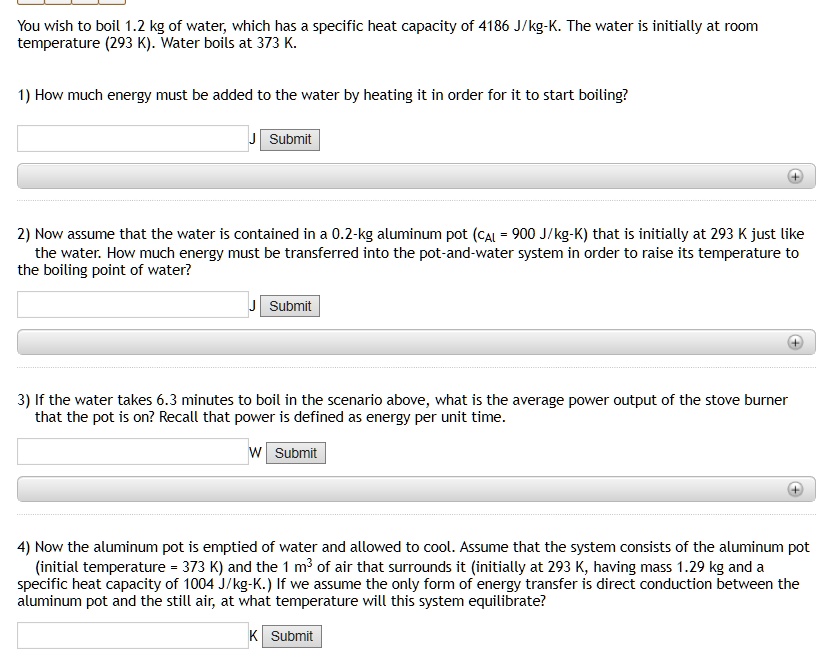
SOLVED: You wish to boil 1.2 kg of water; which has specific heat capacity of 4186 JIkg-K. The water is initially at room temperature (293 K) Water boils at 373 K 1)

Water is brought to boil under the pressure of 1.0 atm . When an electric current of 0.50 A from a 12 V supply is passed for 300 s through resistance in

Normal boiling point of water is 373 K. Vapour pressure of water at 298 K is23 mm enthalpy of va... - YouTube