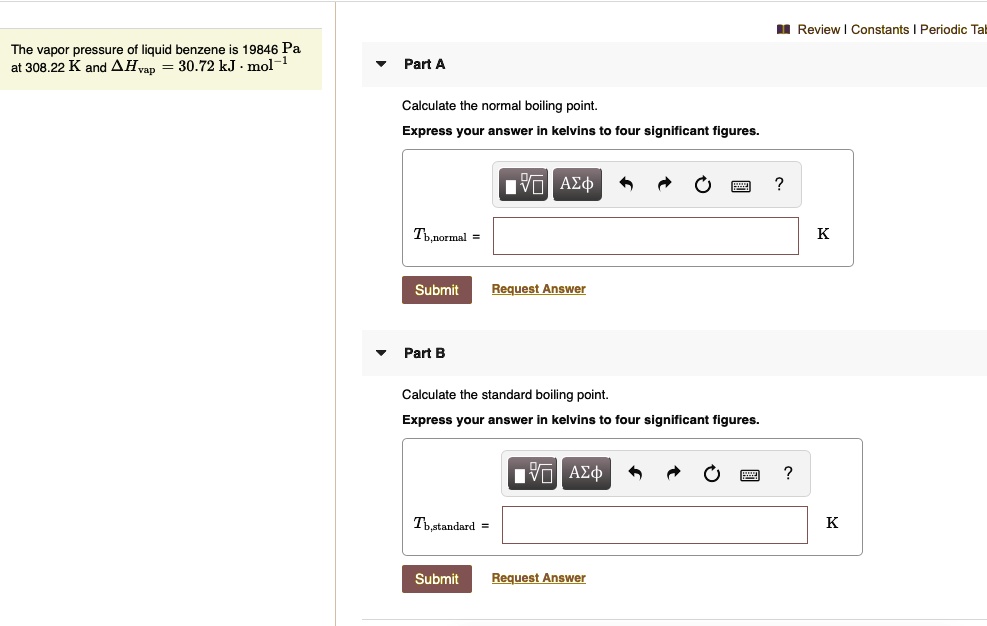
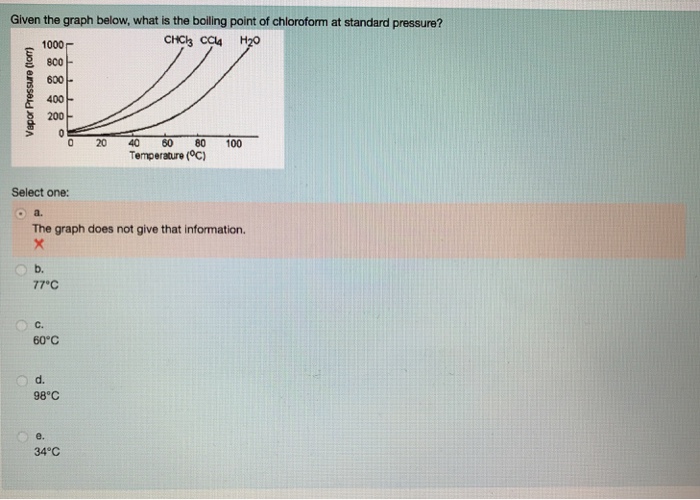
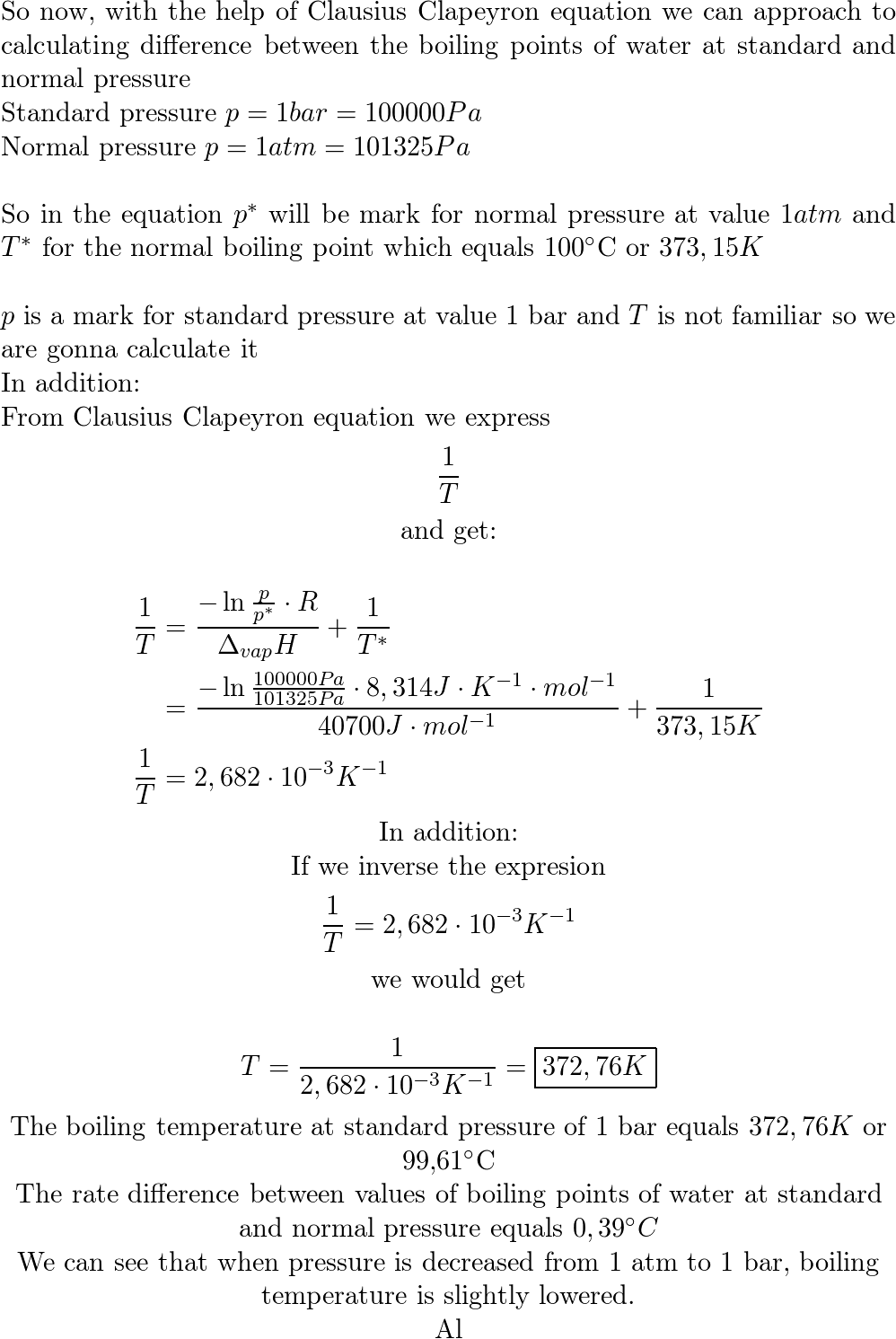
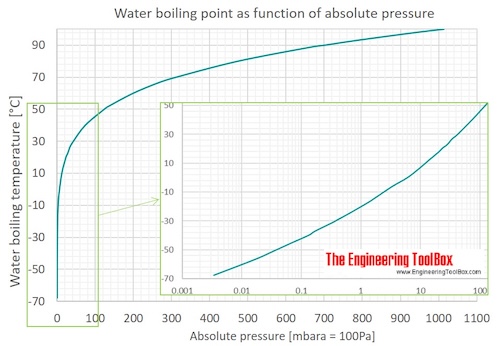
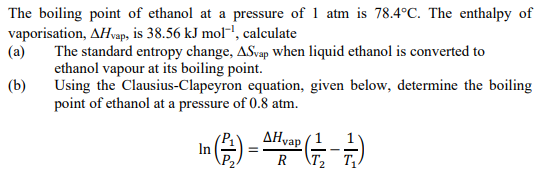
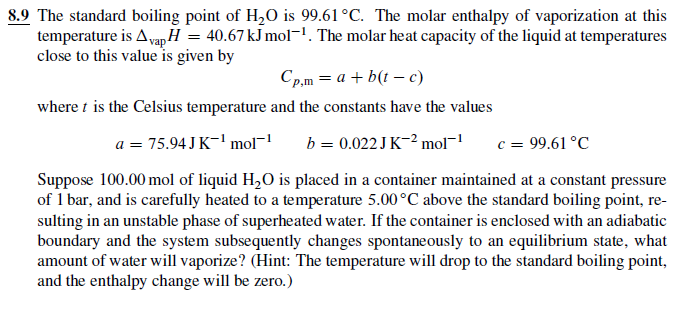
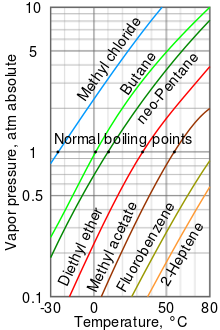
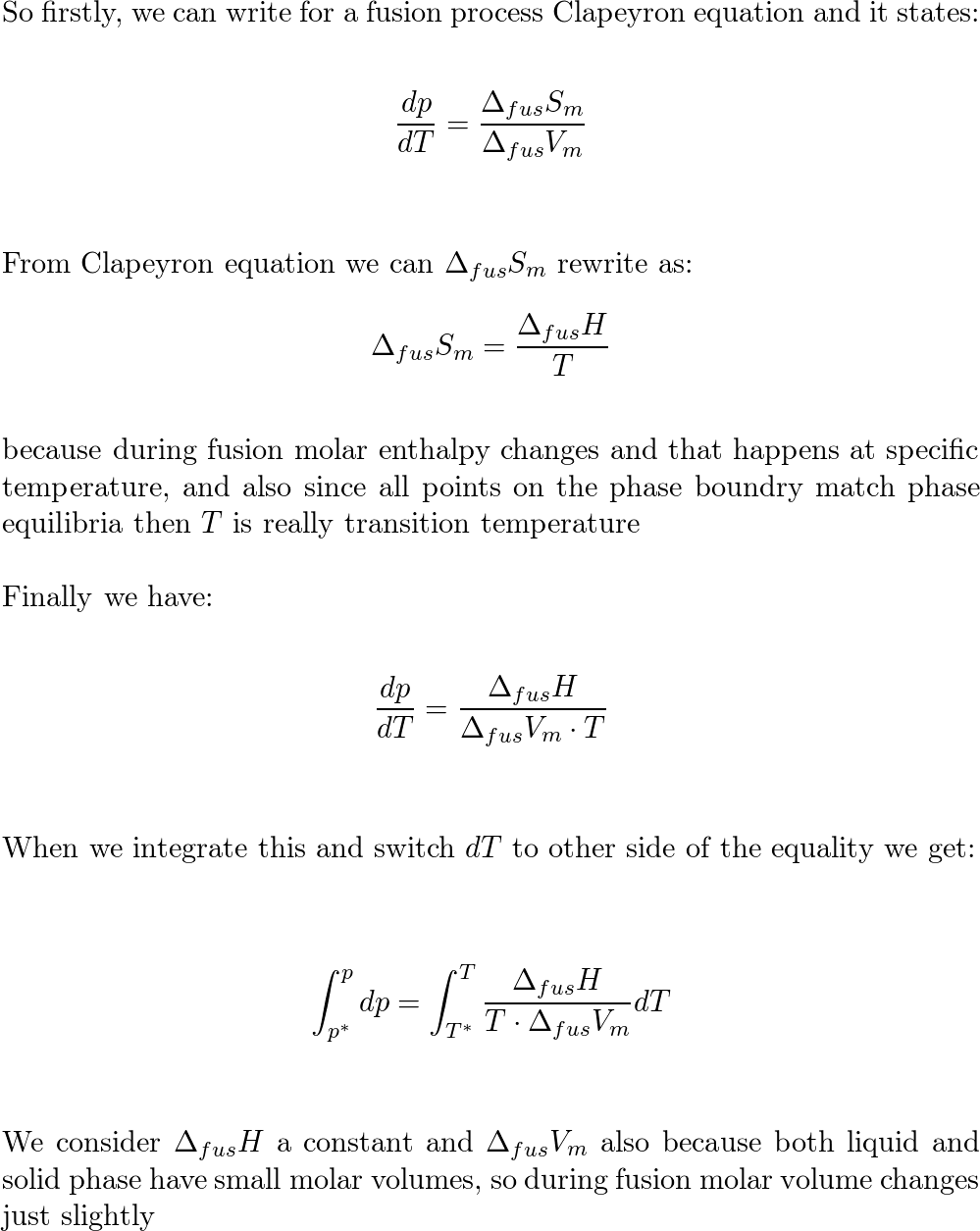Lowering of vapour pressure of 1.00 m aqueous solution of a non volatile solute in hypothetical solvent of molar mass 40g at its normal boiling point is:

what is difference between normal boiling point and standrad boiling point?3 points required ,class 11 - Brainly.in

Given the following data, estimate the boiling point of carbon disulfide, CS2 , assuming that ?So and ?Ho are temperature-independent. Table showing the endothermic heat and the Standard entropy for | Homework.Study.com

Difference Between Normal Boiling Point and Standard Boiling Point | Compare the Difference Between Similar Terms

SOLVED: The boiling point of methanol is 65.0^∘C and the standard enthalpy of formation of methanol vapor is -201.2 kJ / mol . Calculate the vapor pressure of methanol (in mmHg )

A substance P has a standard boiling point of 450K. Which of the following options contain correct - YouTube