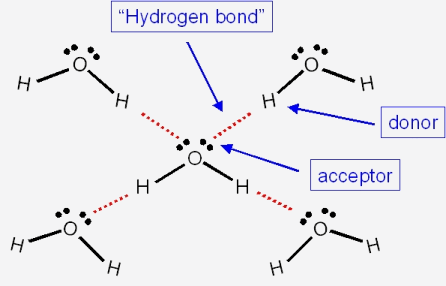
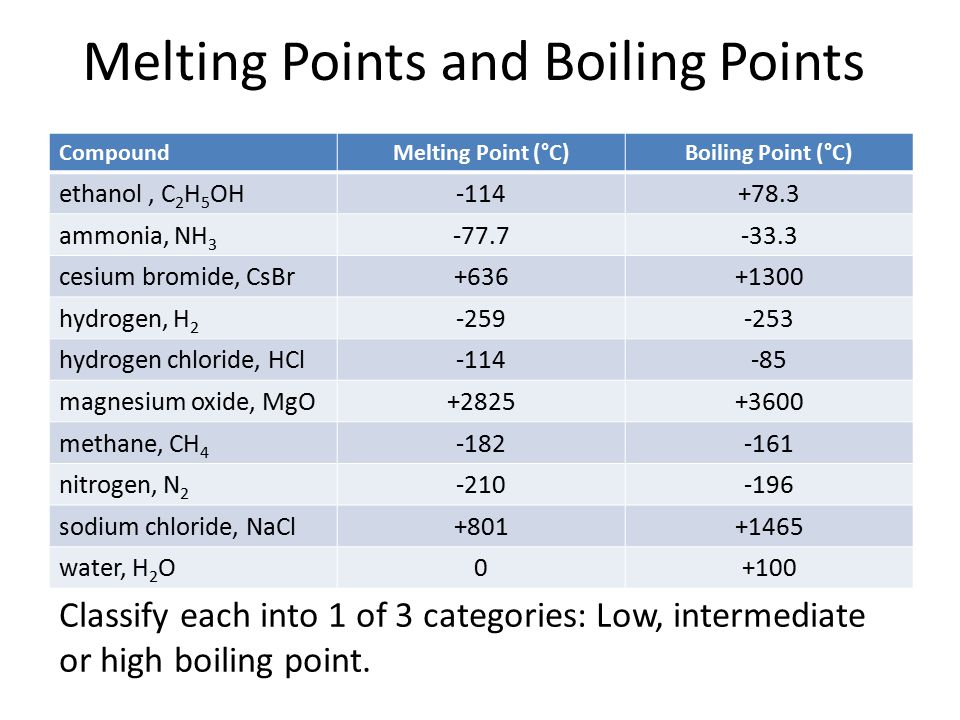
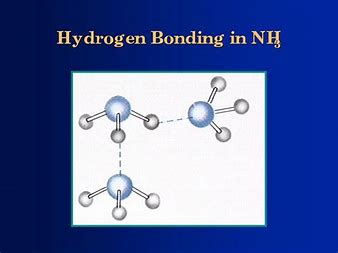
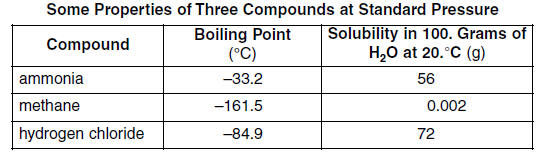
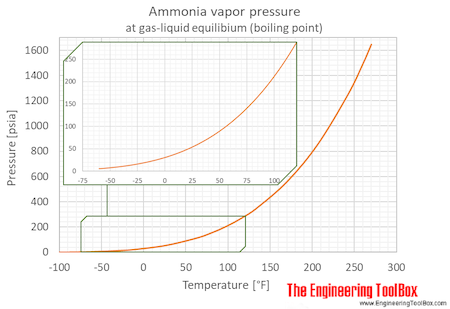
The boiling point of phosphine, PH3 (-88 degrees C) is lower than that of ammonia, NH3 (-33 degrees C) even though phosphine has twice the molar mass of NH3. Why? | Homework.Study.com
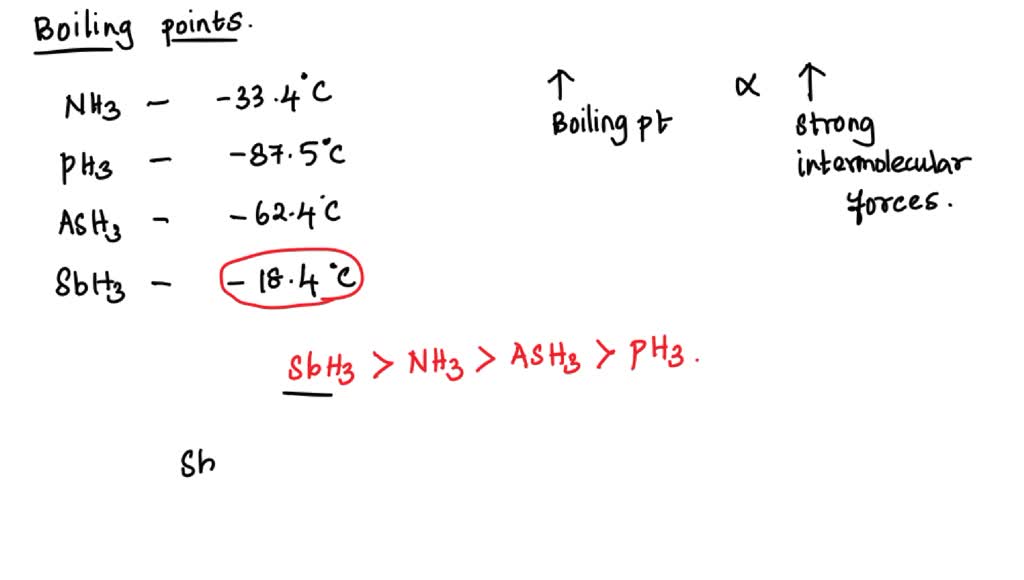
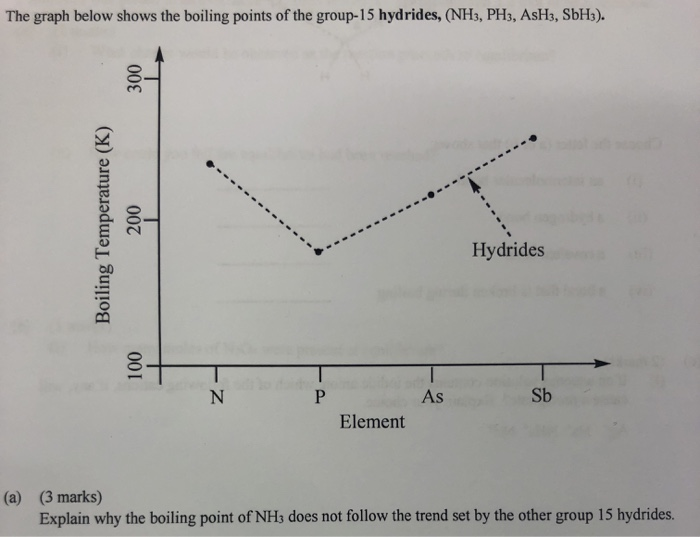
SOLVED: The boiling point of NH3, PH3,AsH3 and SbH3 are respectively -33.4 oC,-87.5 oC, -62.4 oC, -18.4oC. Explain the variation of their boiling points in terms of the types of intermolecular forces.

The hydrides of group 5A are NH3, PH3, AsH3, and SbH3. Arrange them from highest to lowest boiling point. - Brainly.com
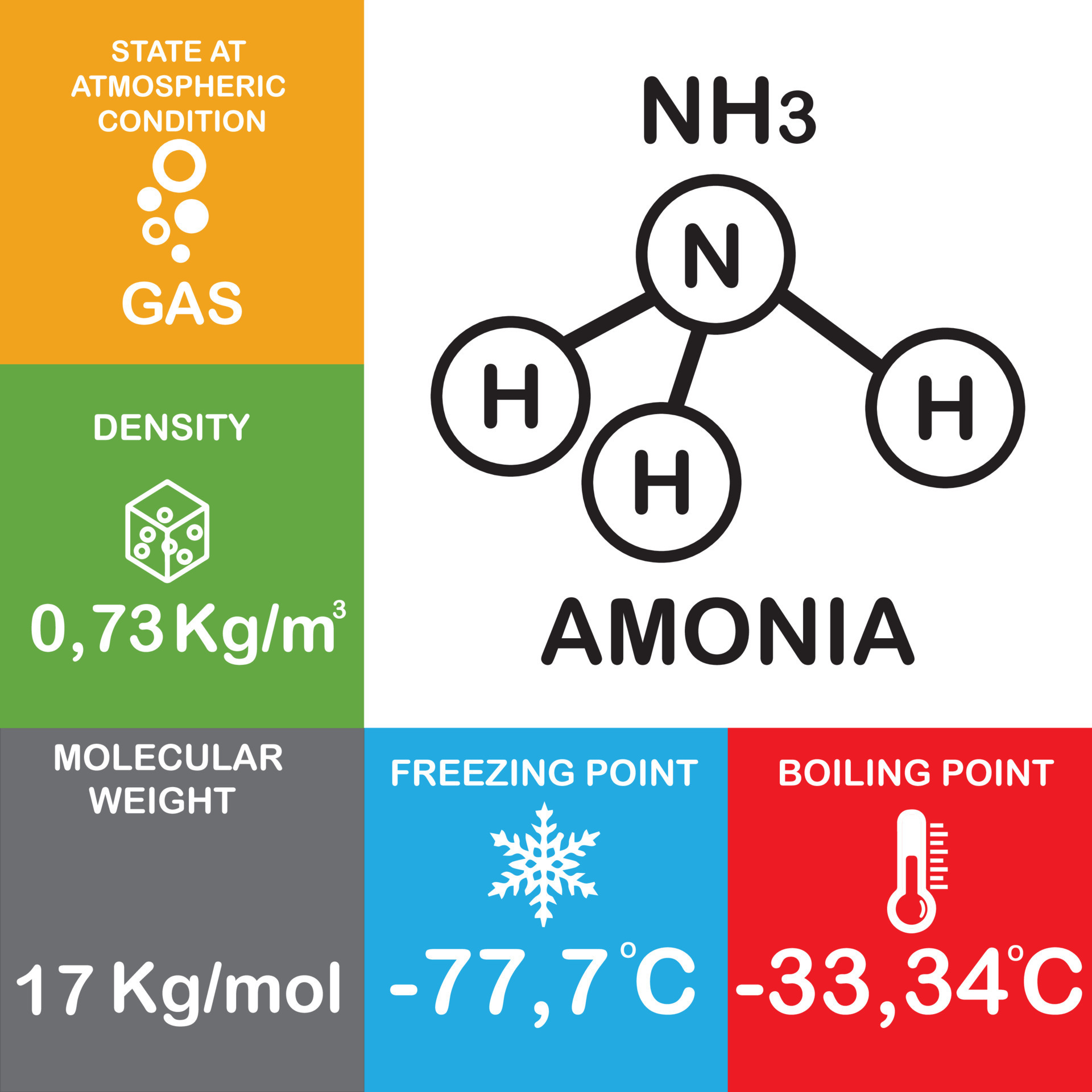
NH3 or amonia structure molecule and atom, molecule Properties and Chemical Compound Structure water consist of boiling point, phase, density, freezing point and molecular weight gas 15324156 Vector Art at Vecteezy