EP0183110B1 - Azeotrope-like compositions of trichlorotrifluoroethane, ethanol, acetone, nitromethane and hexane - Google Patents

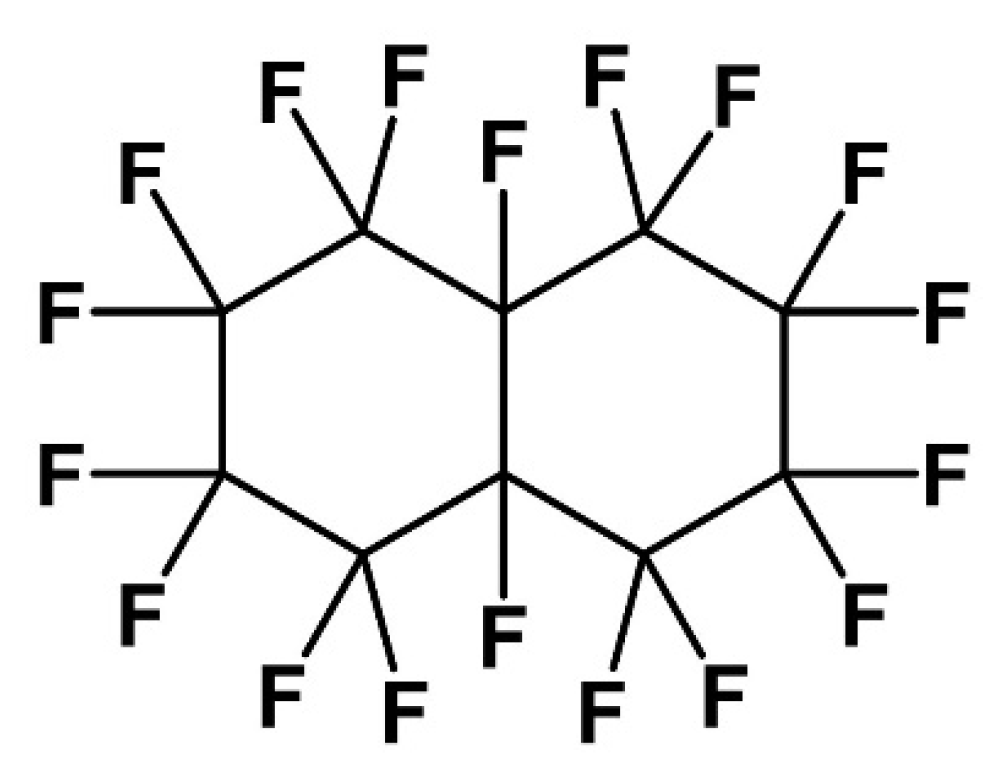
organic chemistry - Why does neopentane have a higher melting point than n-pentane? - Chemistry Stack Exchange

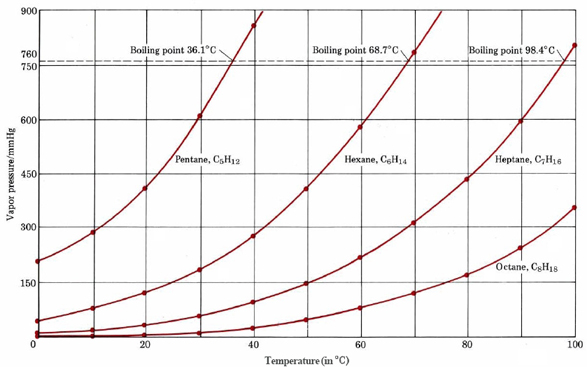
n -Hexane density versus pressure at di ff erent temperature values: 1... | Download Scientific Diagram

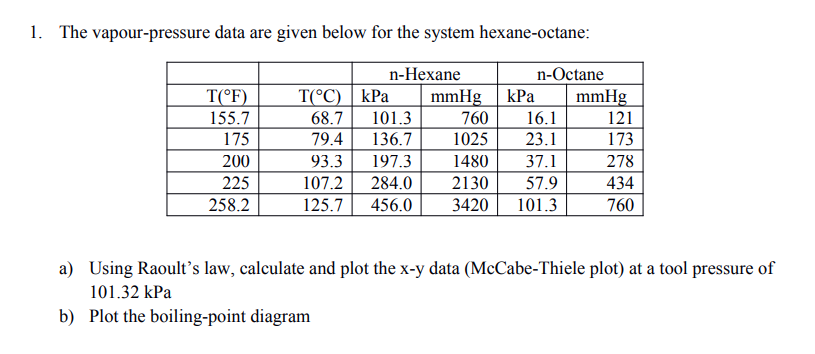
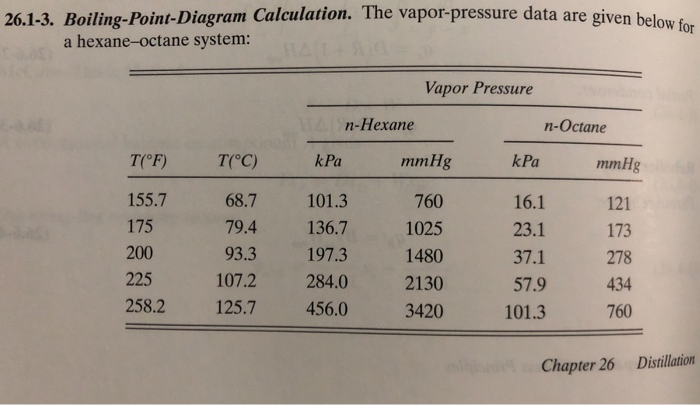
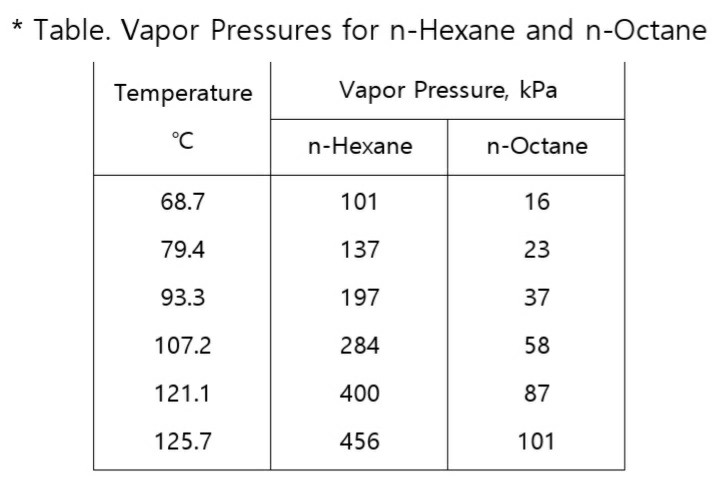
OneClass: For Problems 2 and 3, use the n-hexane, n-octane data from Problem 1. Number 1 answer is sh...

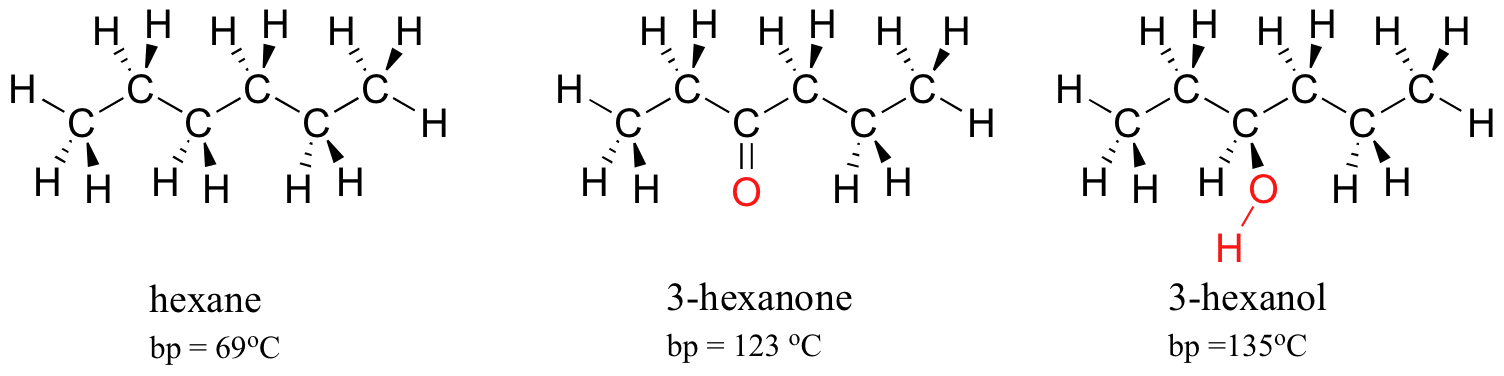
organic chemistry - Why do cyclic hydrocarbons have higher boiling points than their acyclic isomers? - Chemistry Stack Exchange