A solution M is prepared by mixing ethanol and water. The mole fraction of ethanol in the mixture is 0.9. Water is added to the solution M such that the fraction of

1 KCl and MgCl2 are strong electrohytes 1m KCl solution elevates the boiling point by 0 6 K - Chemistry - Solutions - 14898411 | Meritnation.com
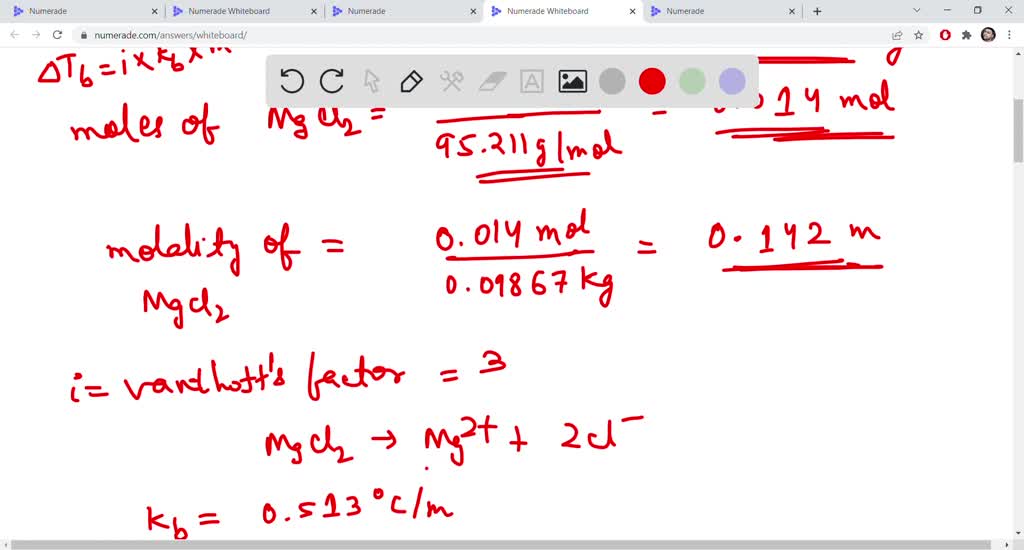
SOLVED: the boiling point ofa 1.33 % by mass magnesium chloride solution Express your answer using two decimal places:

OneClass: Calculate the freezing point and boiling point of each of the following solutions. a. 0.050...

The elevation in boiling point of a solution of 9.43g of MgCl2 in 1kg of water is : ( Kb = 0.52K kg mol^-1 , Molar mass of MgCl2 = 94.3g mol^-1 )
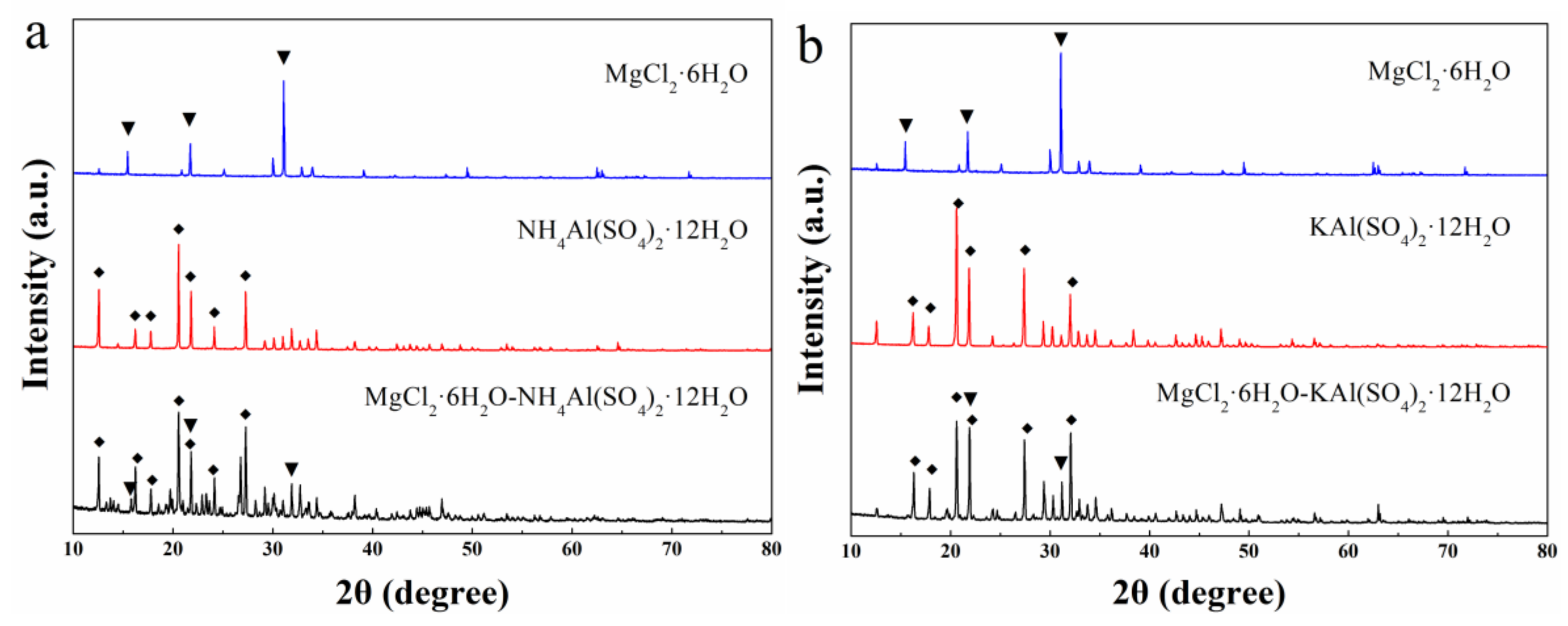
Molecules | Free Full-Text | Compounding MgCl2·6H2O with NH4Al(SO4)2·12H2O or KAl(SO4)2·12H2O to Obtain Binary Hydrated Salts as High-Performance Phase Change Materials

a) Calculate the freezing point of the solution when 1.9 g of MgCl2 (M = 95 g mol−1) was dissolved in 50 g of water, assuming MgCl2 undergoes complete ionization. (Kf for

The elevation in boiling point of a solution of 9.43g of MgCl2 in 1kg of water is : ( Kb = 0.52K kg mol^-1 , Molar mass of MgCl2 = 94.3g mol^-1 )

Stability diagram of magnesium chloride in solution with water. The... | Download Scientific Diagram

Difference Between Calcium Chloride and Magnesium Chloride | Compare the Difference Between Similar Terms
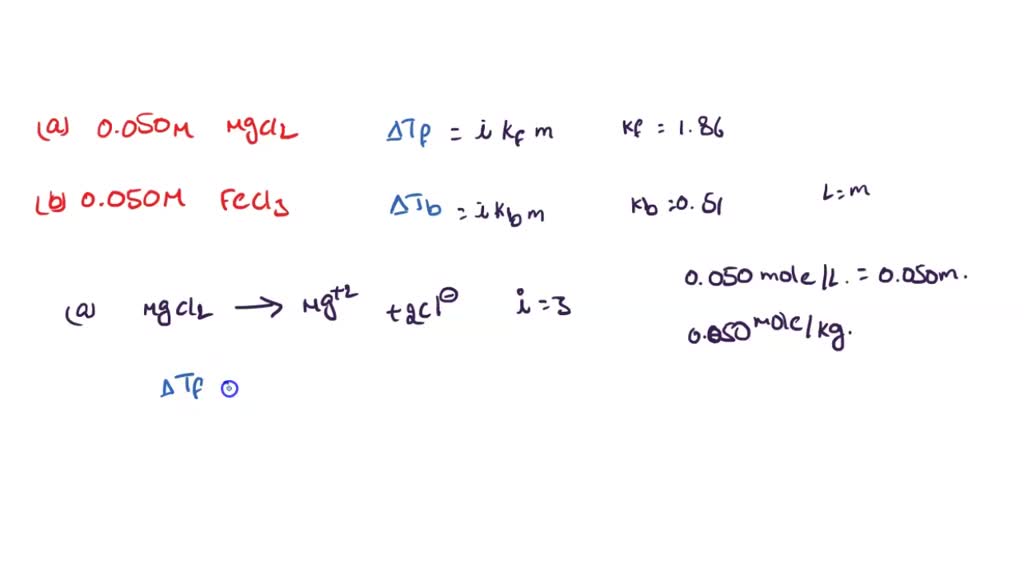
SOLVED: Estimate the freezing and boiling points of the following solutions: a. 0.050 M MgCl2 b. 0.050 M FeCl3