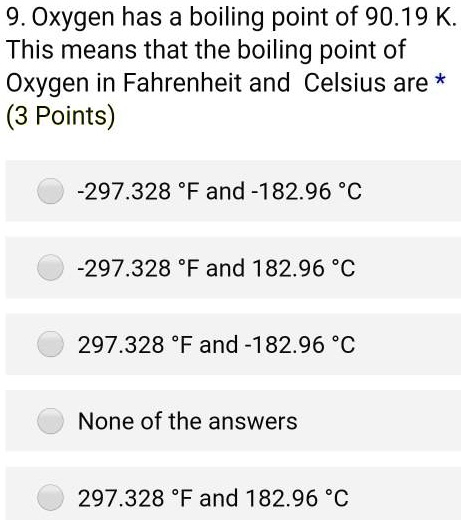
The normal melting and boiling points of a substance are -163 degrees Celsius and -128 degrees Celsius, respectively. Its triple point is at 125 K and 0.37 atm. Its critical point is

Temperature Temperature Scales Fahrenheit 212 o F 180 o F 32 o F Celcius 100 o C 0 o C Kelvin 373 K 100 K 273 K Boiling point of water Freezing point. - ppt download

Determine the normal boiling point in K of a substance whose vapor pressure is 55.1 mmHg at 23.2^o C and has a ?H_vap of 32.1 kJ/mol. | Homework.Study.com
Boiling point of water. Fluid boiling with flame in the stove, evaporating in the glass container. Cooker fire. Liquid bubbles. 100 Celsius, 212 Fahrenheit, 273.15 K . Education illustration Vector Stock-Vektorgrafik | Adobe Stock

The normal boiling point of toluene is 110.7^(@)C and its boiling point elevation constant 3.32 " K kg mol"^(-1). The enthalpy of vaporization of toluene is nearly :