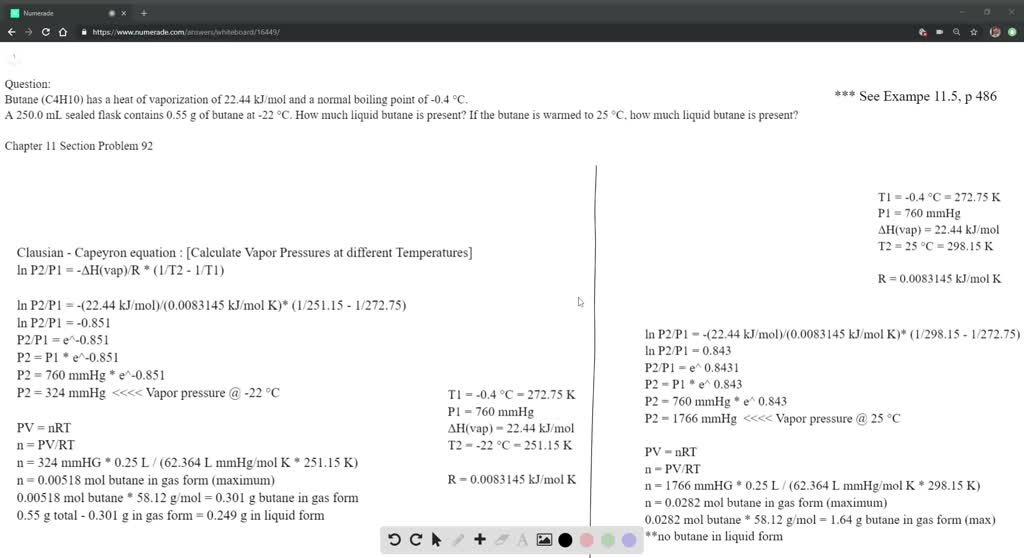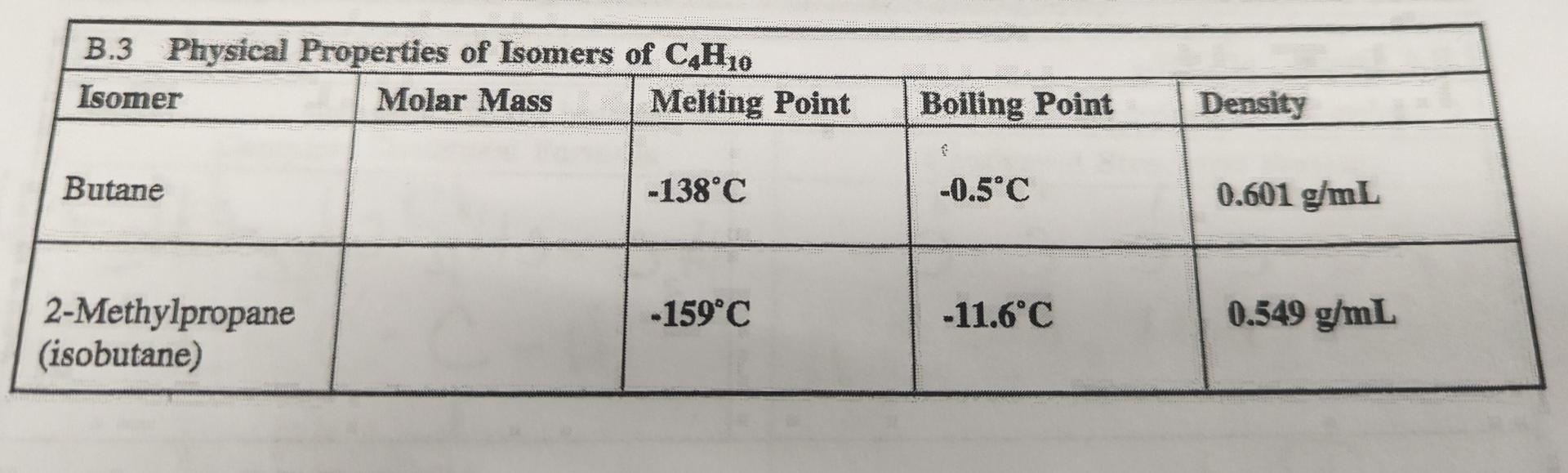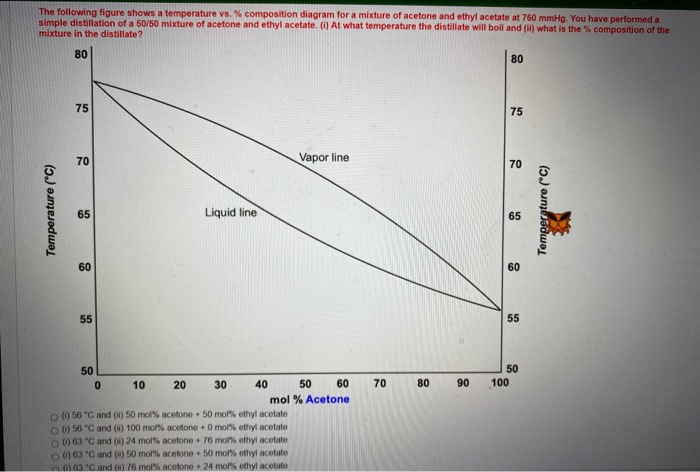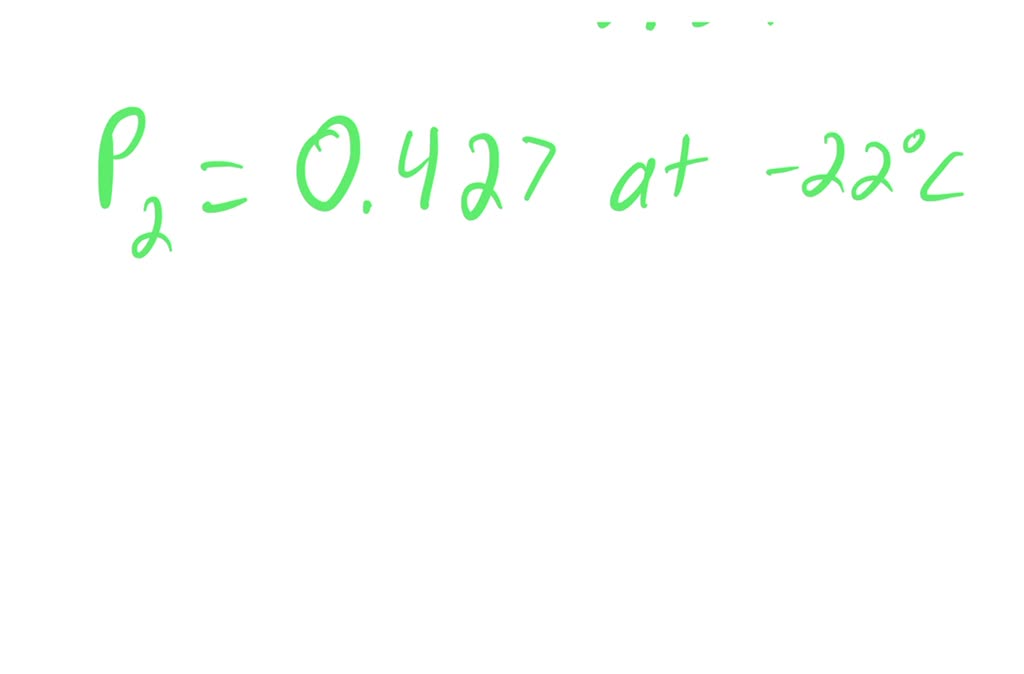
SOLVED: Butane (C4H10) has a heat of vaporization of 22.44 kJ/mol and a normal boiling point of -0.4 ∘C. A 250 mL sealed flask contains 0.55 g of butane at −22∘C. Part
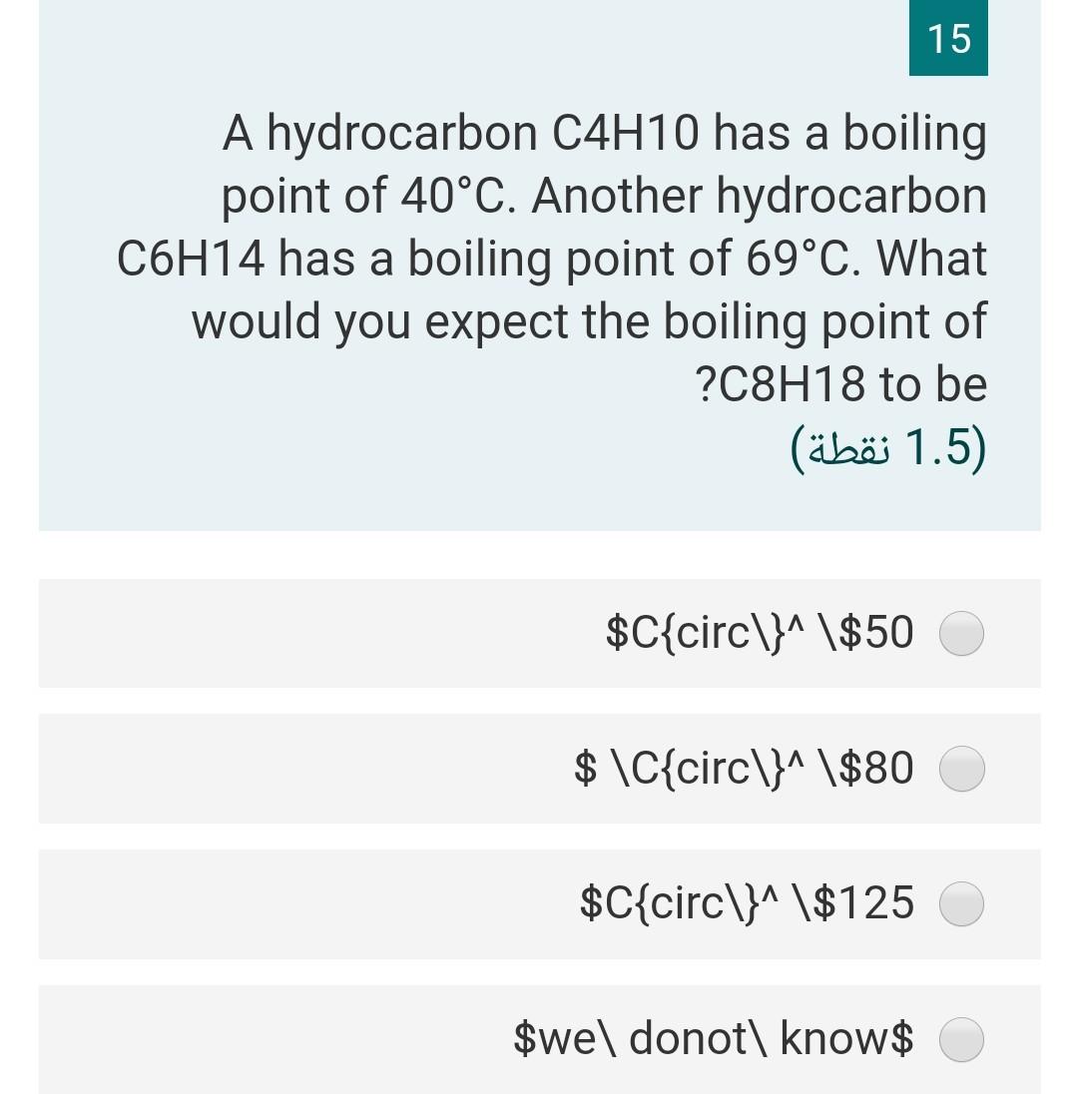
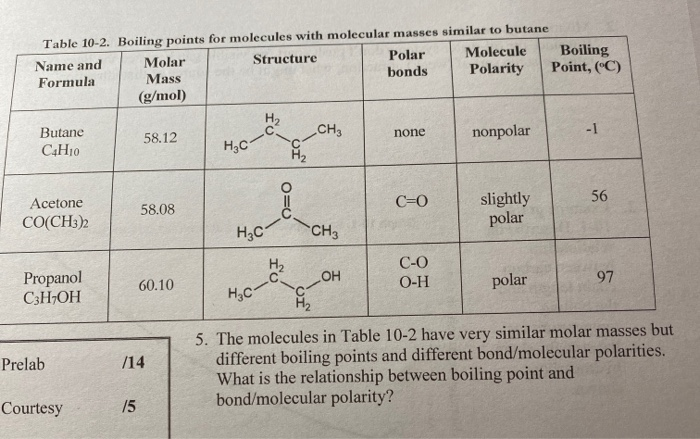
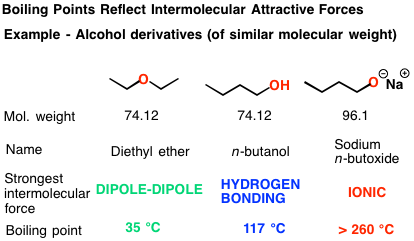
What will be the boiling point of the next alkane, alkene, and alkyne? Will the boiling point of each hydrocarbon be higher or lower? - Quora

Isobutane R600A C4H10 Refrigerant for High and Medium Temperature Applications Suppliers, Manufacturers, Factory - Buy Cooling Agent, Price & Quotation - Juda Trading

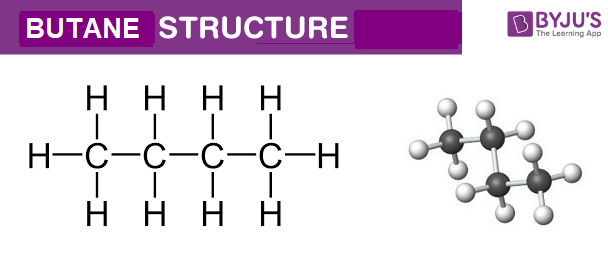
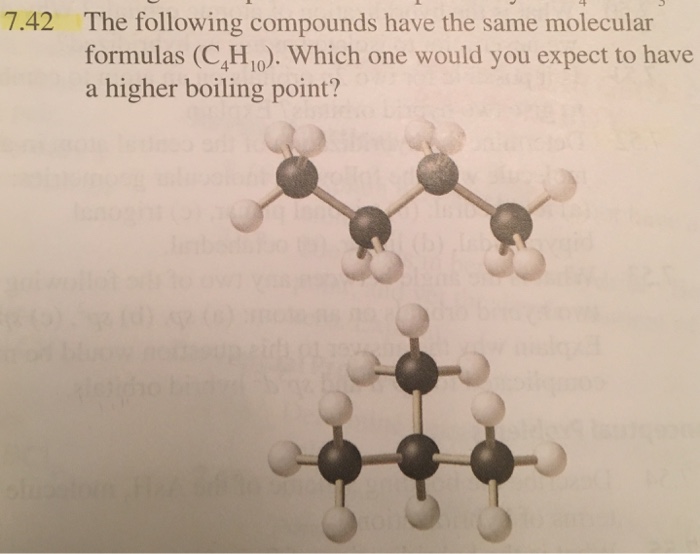
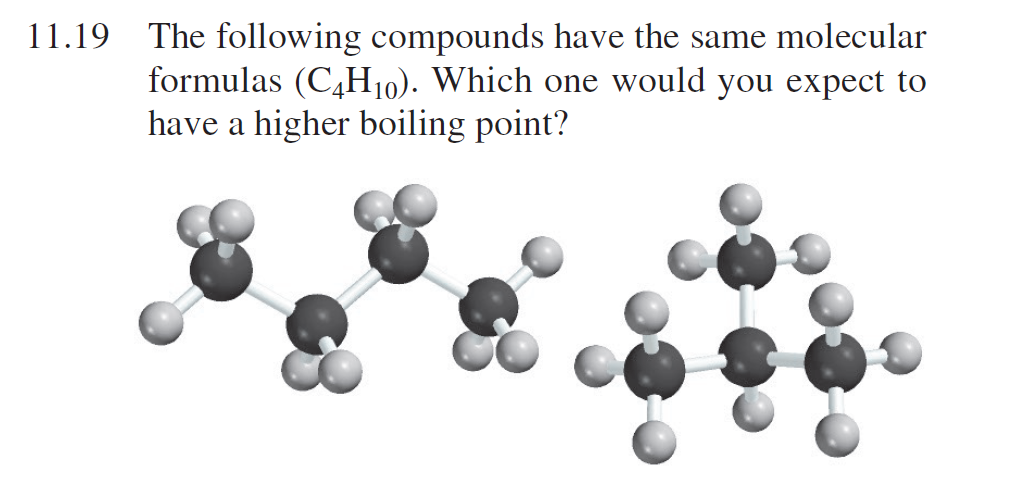
SOLVED: What is the structure for two constitutional isomers with molecular formula of C4H10. Which would have higher boiling point? Which would be water soluble?