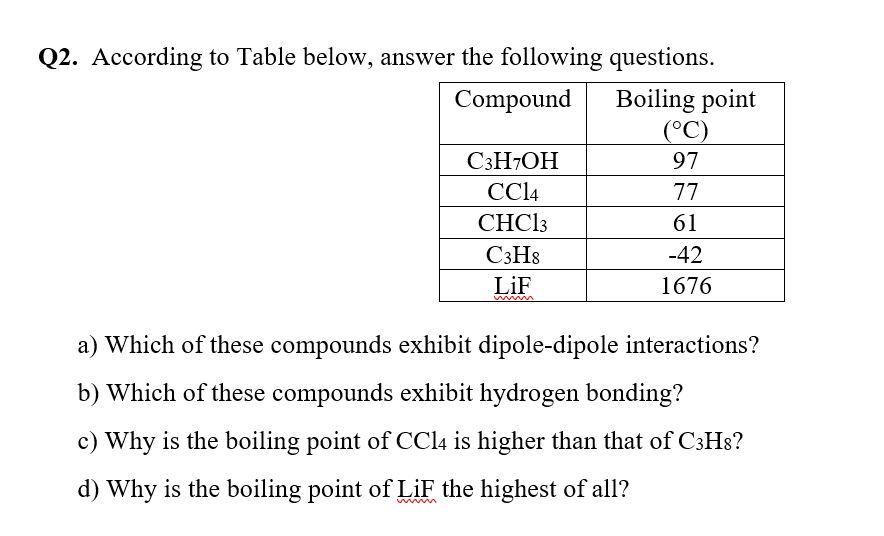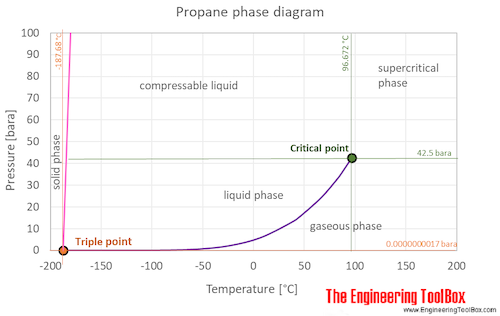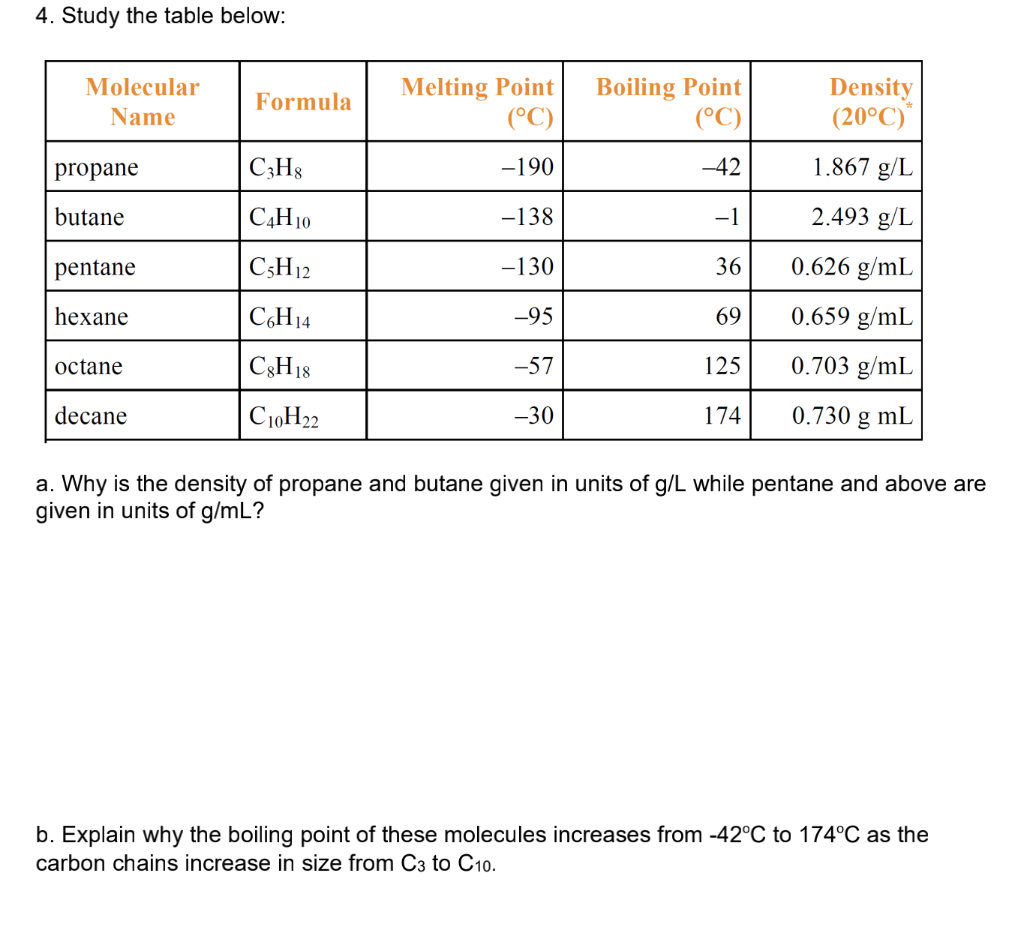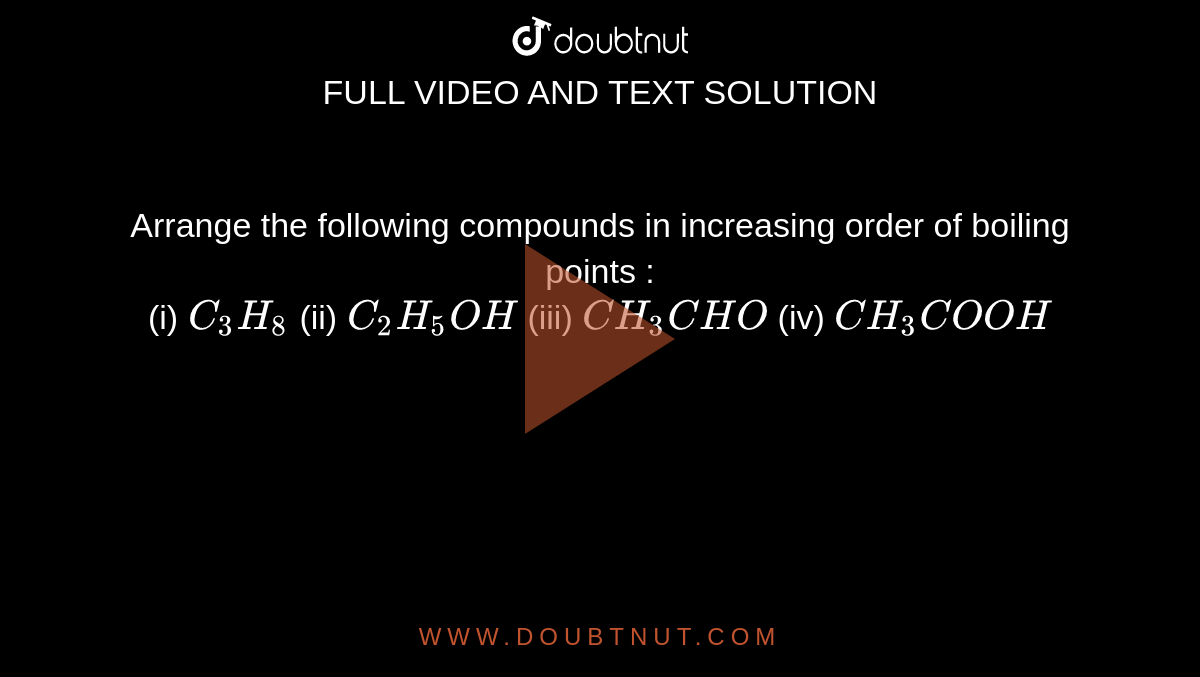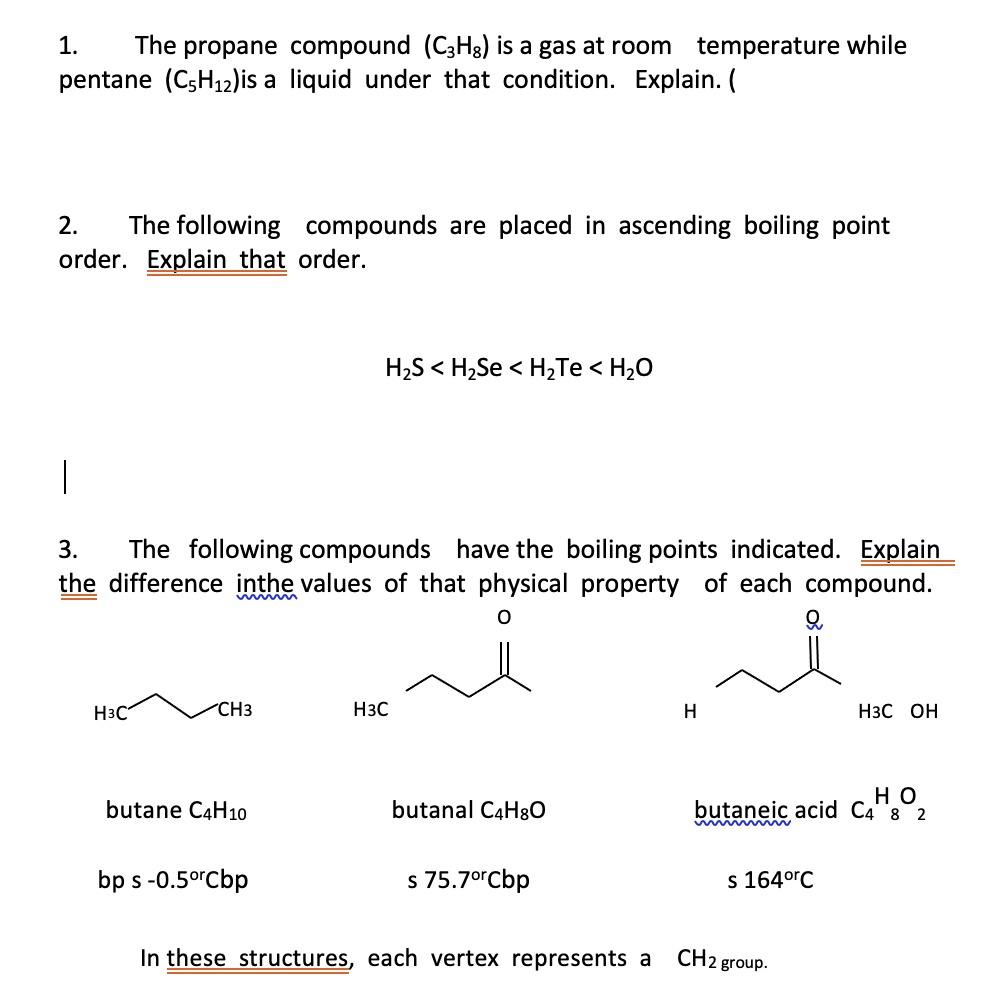
SOLVED: 1. The propane compound (CzHg) is a gas at room temperature while pentane (CsHizlis a liquid under that condition: Explain. 2 The following compounds are placed in ascending boiling point order.

intermolecular forces - How can I determine the highest boiling point given a list of molecules? - Chemistry Stack Exchange
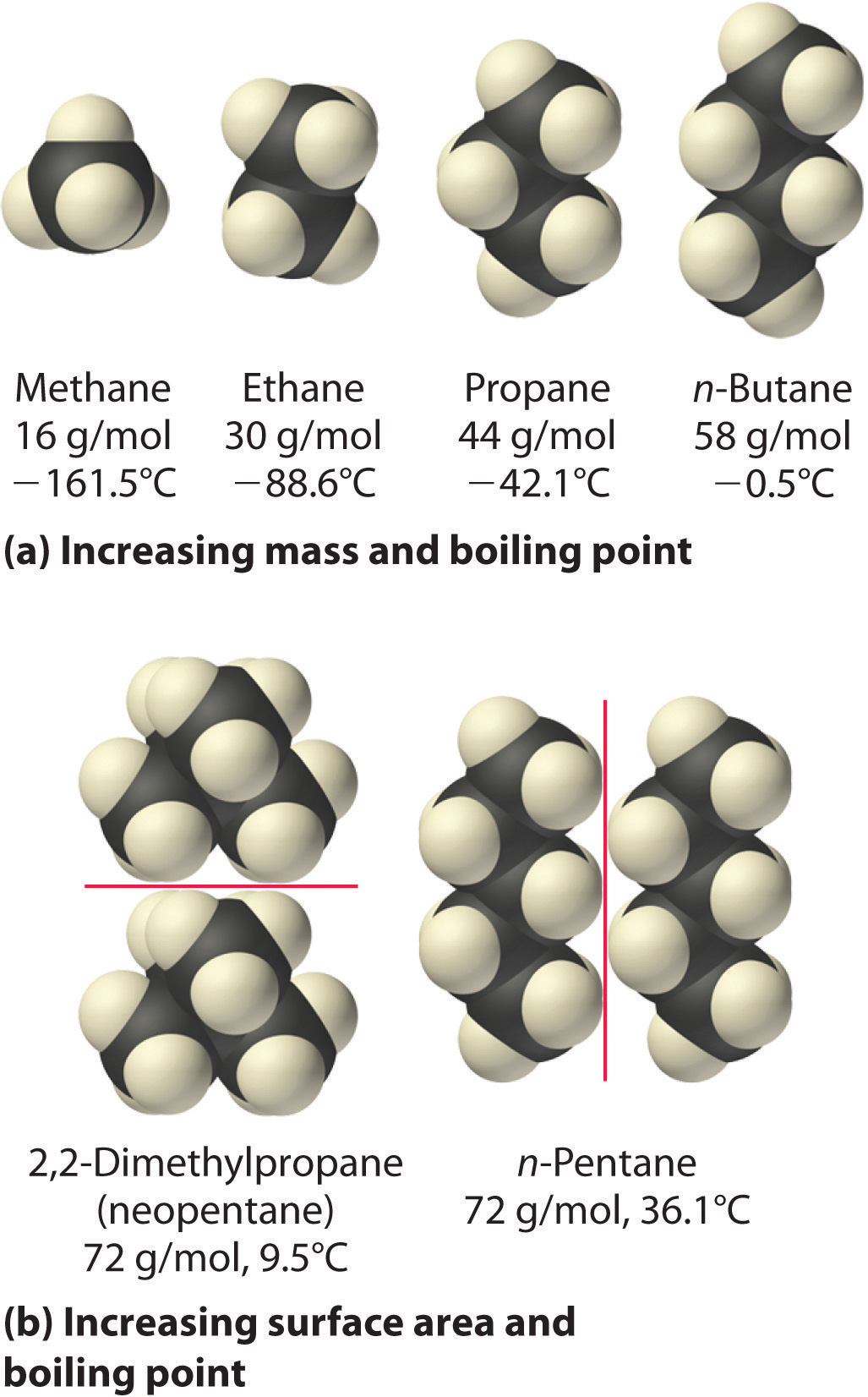
Which molecule would have the largest dispersion molecules forces between other identical? (A) CH4 (B) C3H8 (C) C2H6 (D) C2H4 (E) C4H10 | Socratic

SOLVED: Which of the following will have the highest boiling point? A. C3H8 B. C5H12 C. C6H14 D. CH4
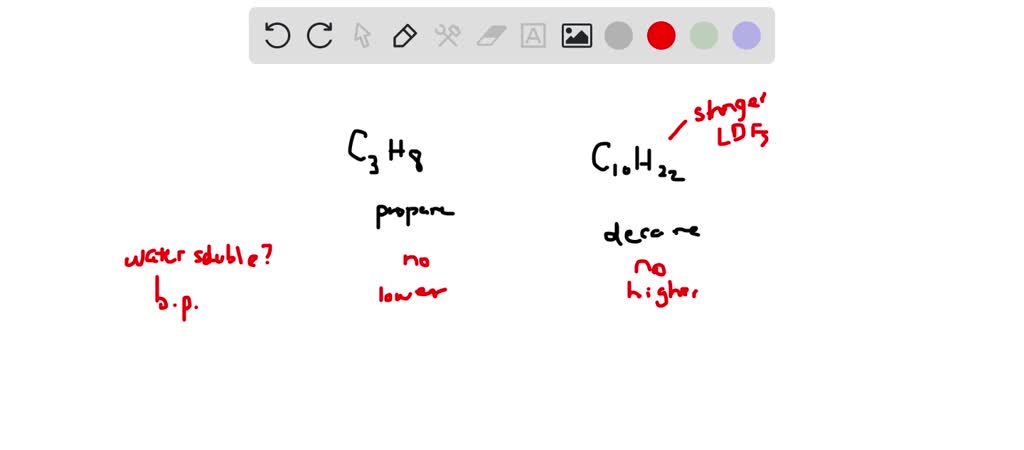
SOLVED: Consider propane (C3H8) and decane (C10H22): Which is soluble in water? Which has lower boiling point? Which has higher boiling point? Which has lower melting point? Which has higher melting point?
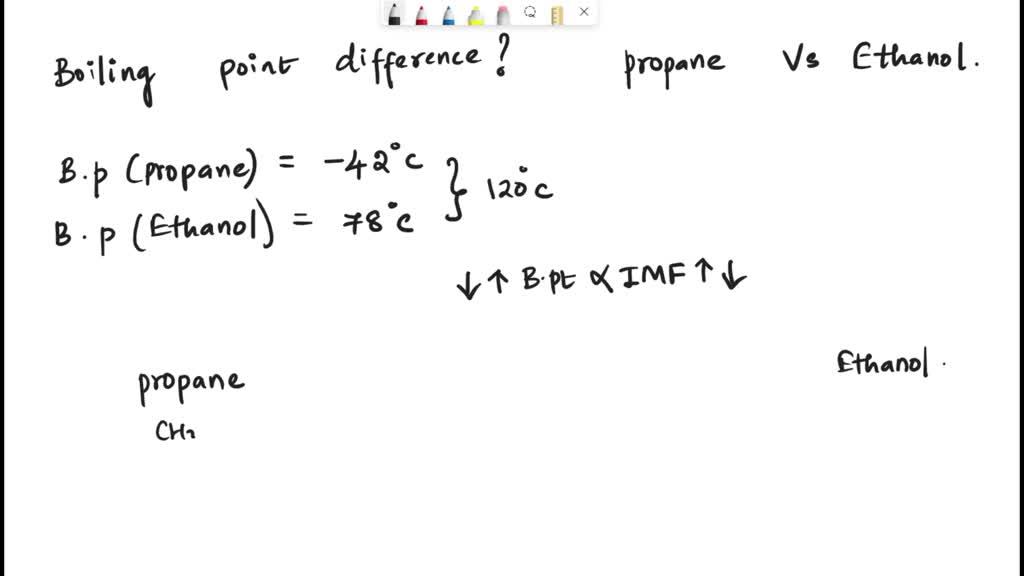
SOLVED: The boiling point of propane (C3H8) is – 42oC and the boiling point of ethanol (C2H6O) is 78oC. Explain the 120oC difference in boiling point using your knowledge of IMF's.

.PNG)