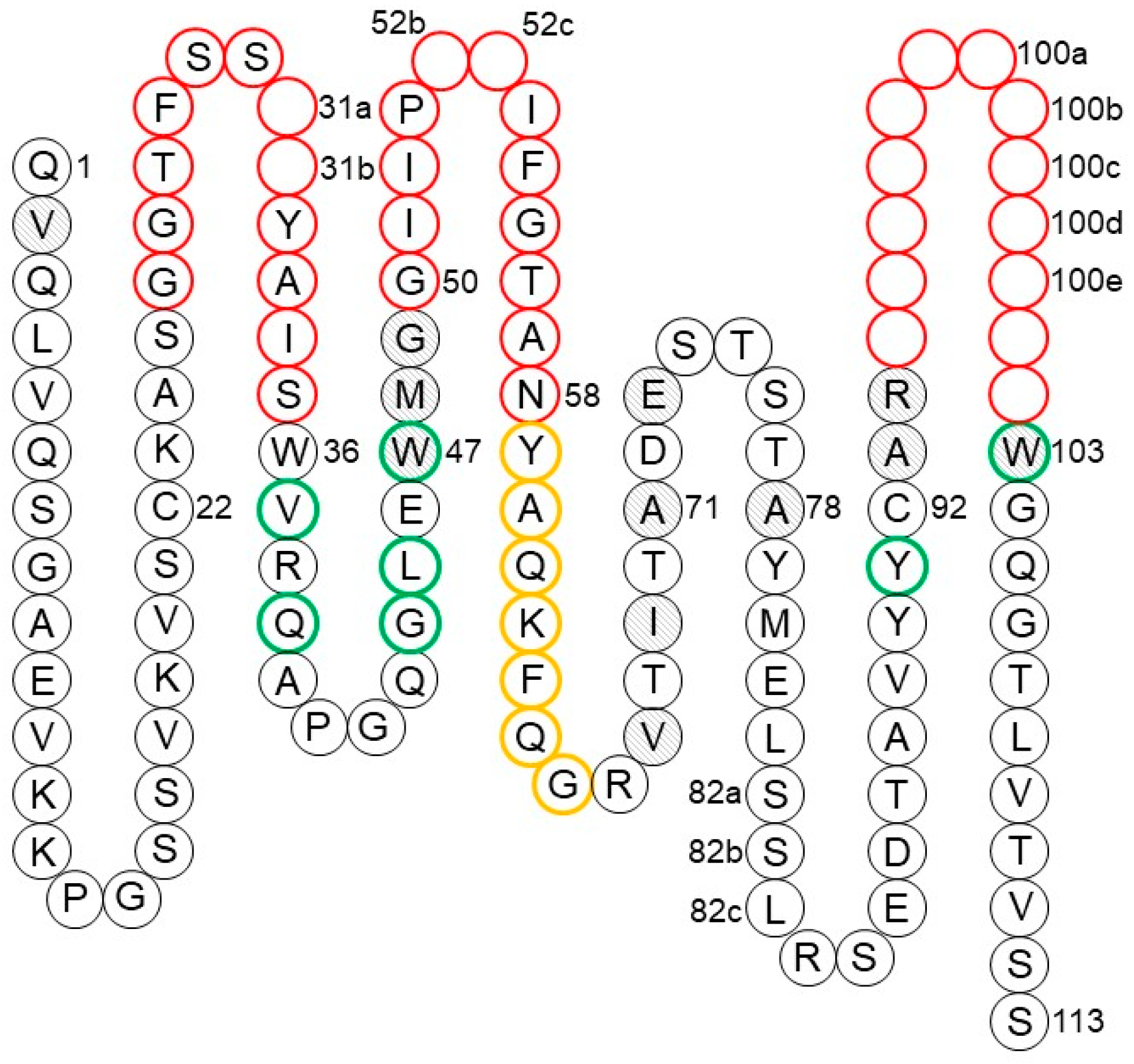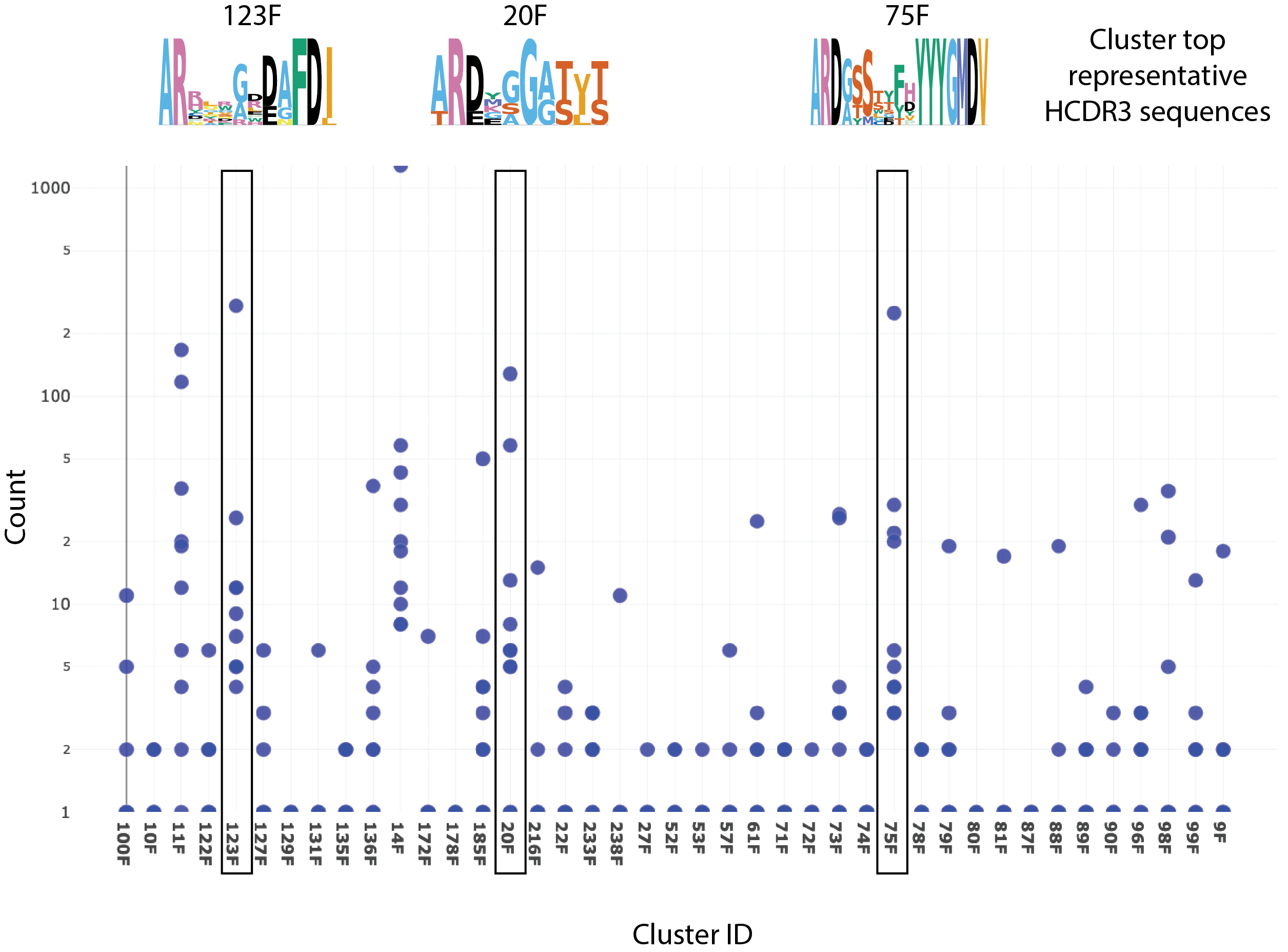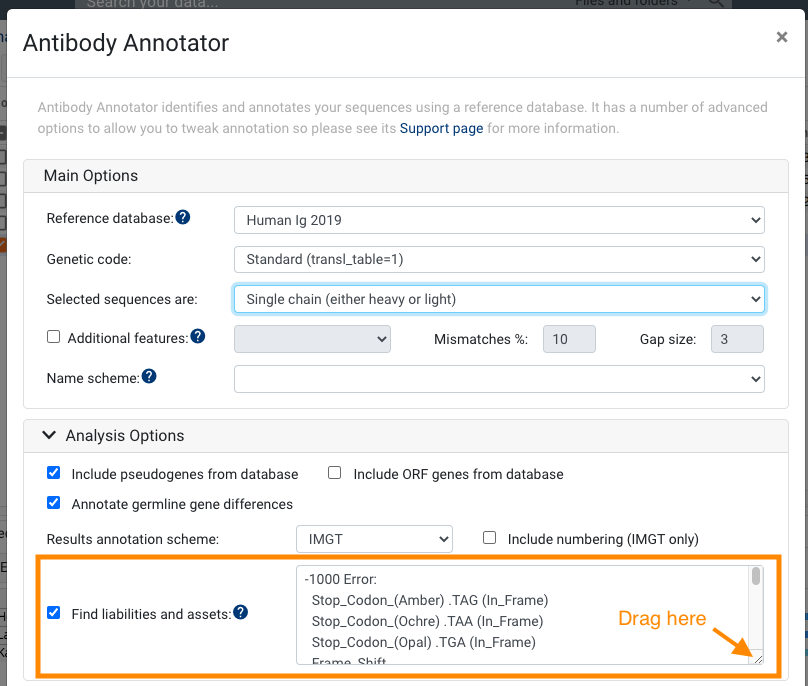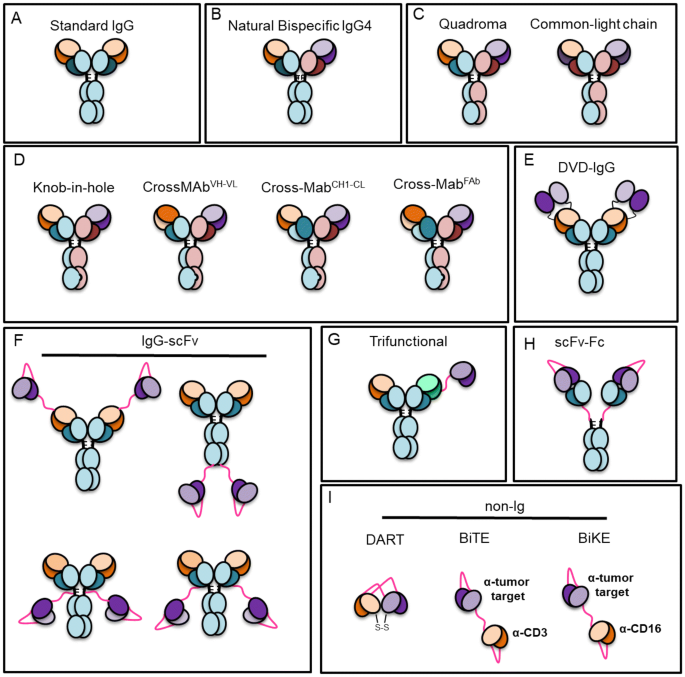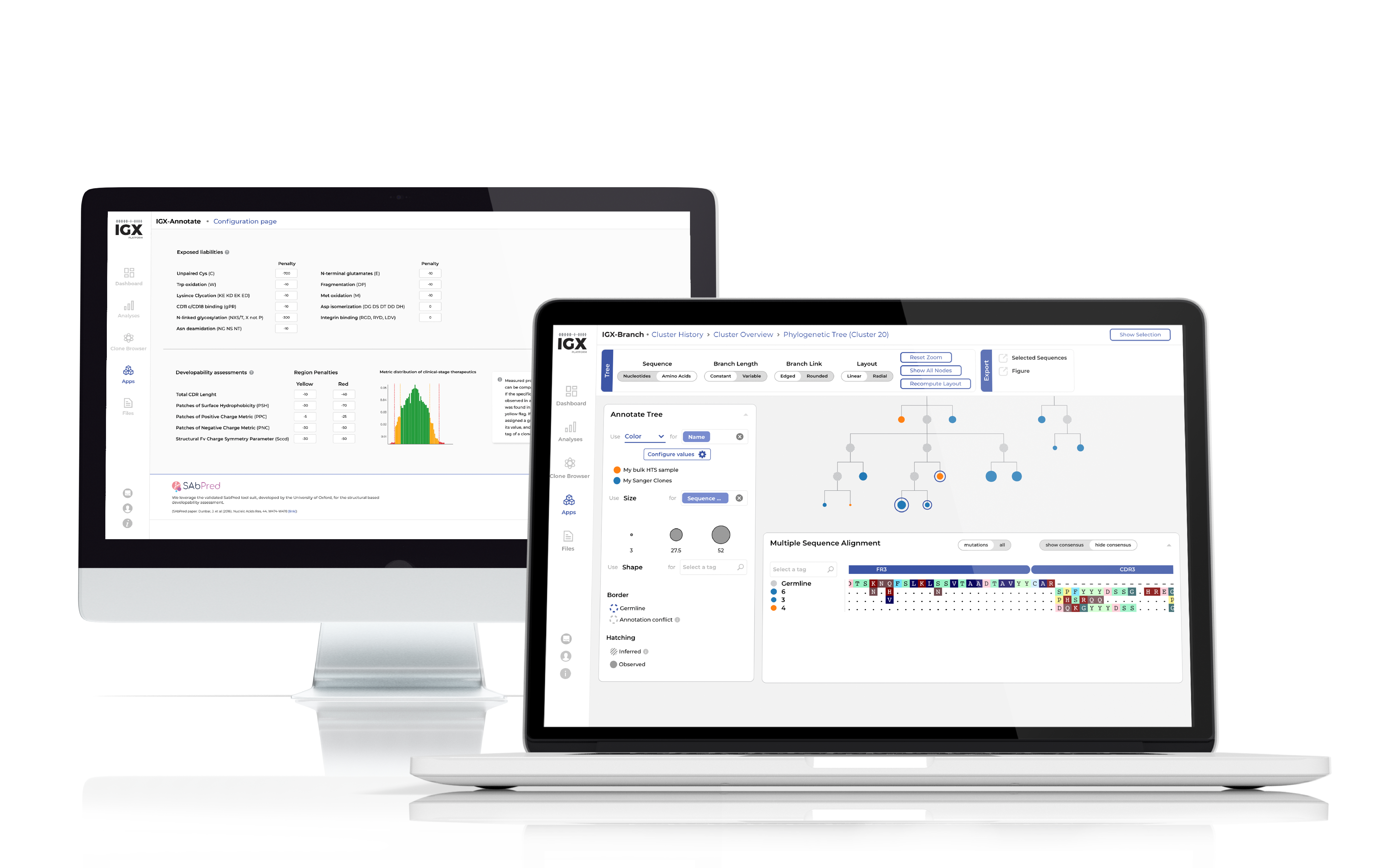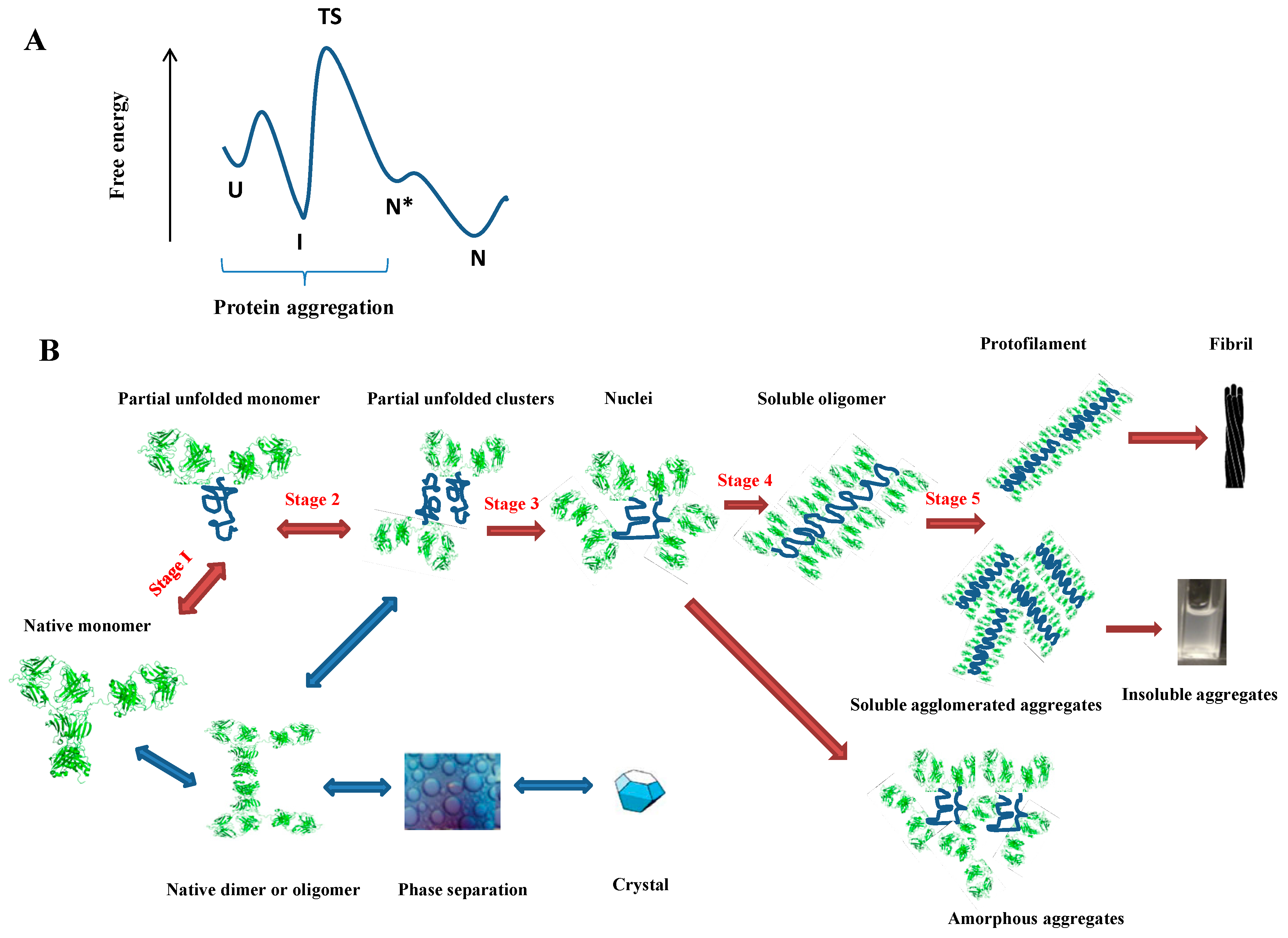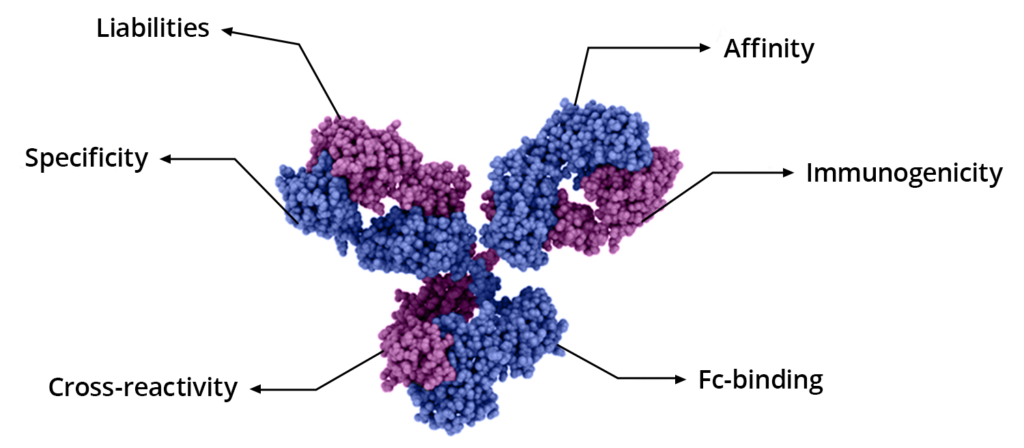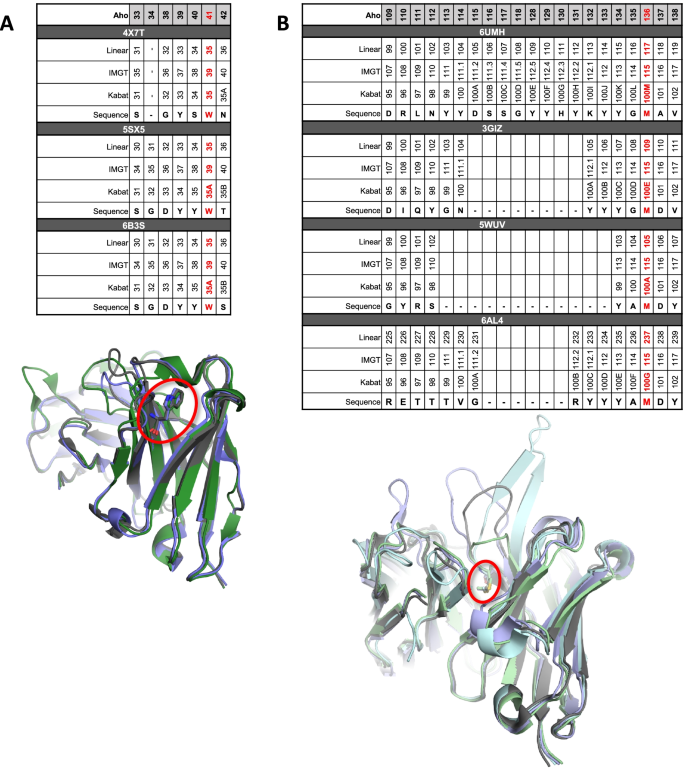
Utilizing cross-product prior knowledge to rapidly de-risk chemical liabilities in therapeutic antibody candidates | AAPS Open | Full Text

Development issues: antibody stability, developability, immunogenicity, and comparability - ScienceDirect

Frequency of liability motifs in all CDRs. Circles show percentages of... | Download Scientific Diagram

Development issues: antibody stability, developability, immunogenicity, and comparability - ScienceDirect
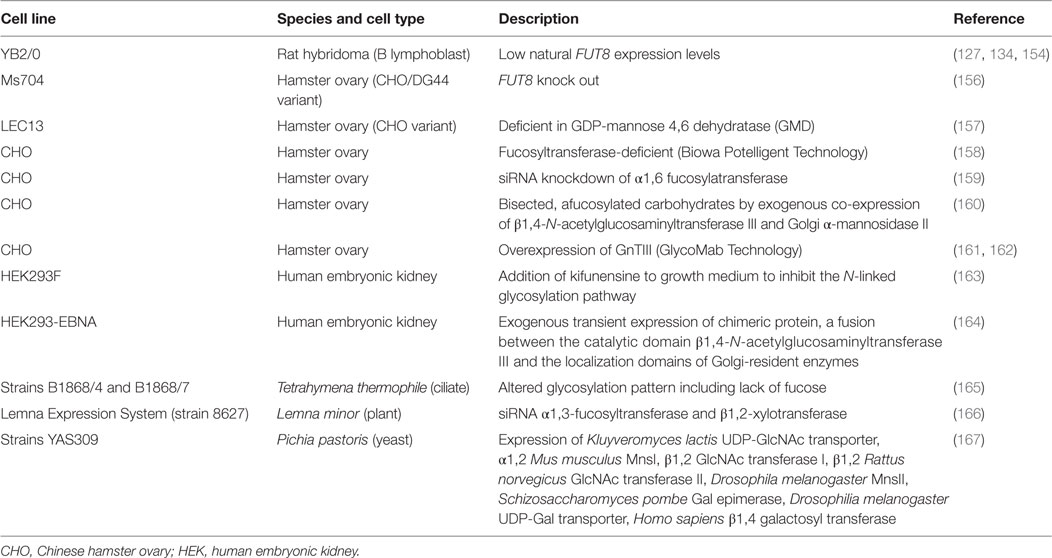
Frontiers | Progress and Challenges in the Design and Clinical Development of Antibodies for Cancer Therapy

Developability Predictions for Antibody Engineering and Risk Mitigation | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology

Oxidation and Deamidation of Monoclonal Antibody Products: Potential Impact on Stability, Biological Activity, and Efficacy - ScienceDirect

Developability Predictions for Antibody Engineering and Risk Mitigation | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology
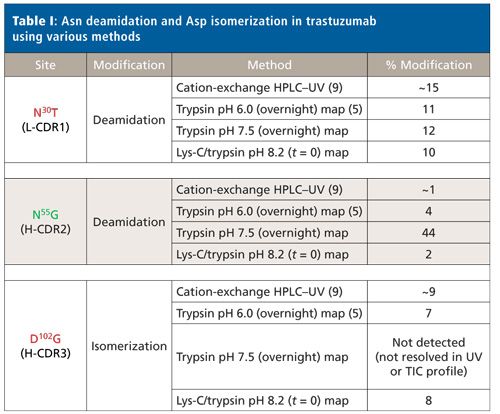
Minimizing Method-Induced Deamidation and Isomerization During Antibody Characterization to Ensure Optimal Understanding of Product Quality Attributes
Drug-like antibodies with high affinity, diversity and developability directly from next-generation antibody libraries
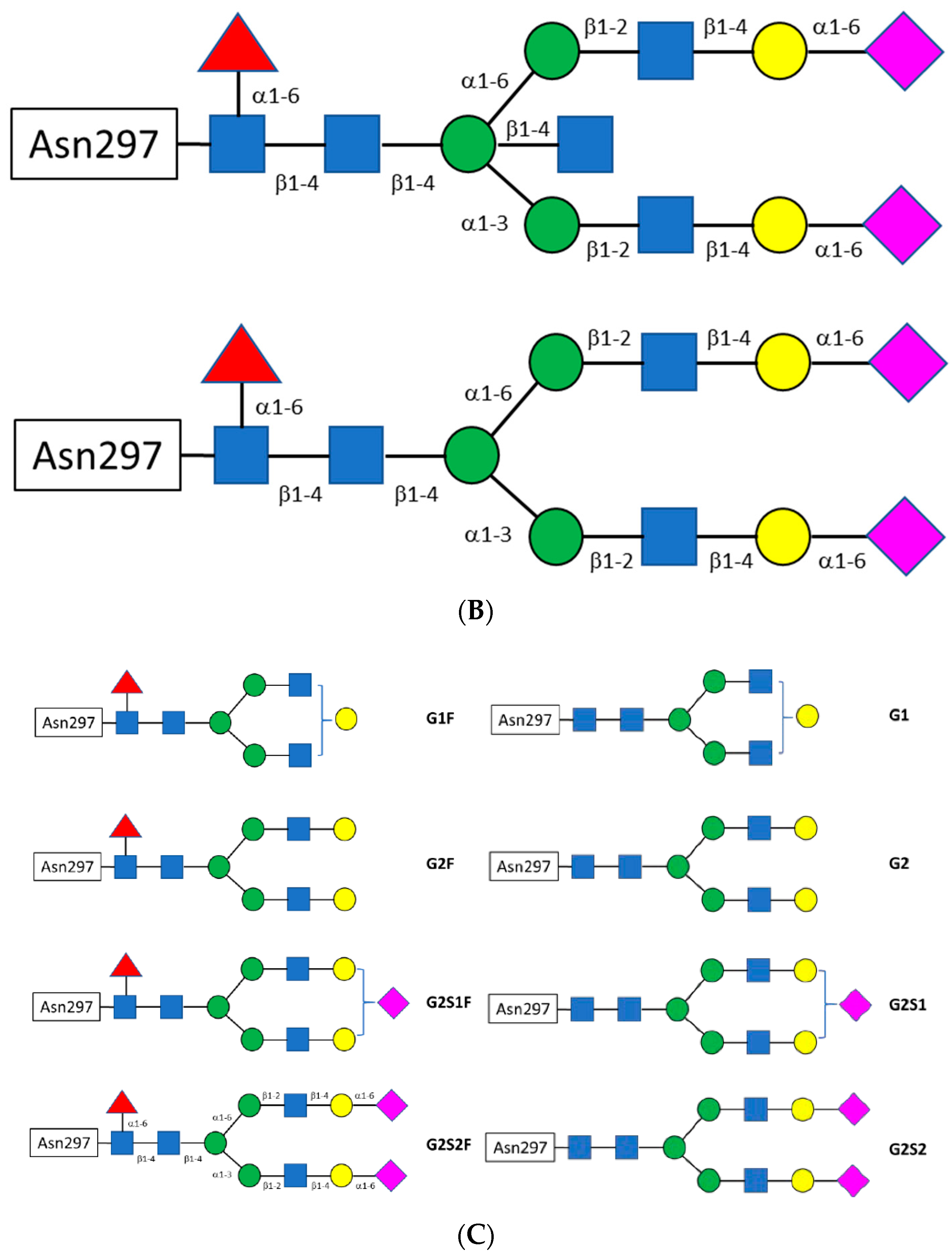
Antibodies | Free Full-Text | Antibody Structure and Function: The Basis for Engineering Therapeutics