Determine the boiling points of 1 m solution of sugar, glucose, urea, sodium chloride, barium chloride, aluminium chloride. | Homework.Study.com

Pressure-temperature phase diagram of AlCl 3 including (extrapolation... | Download Scientific Diagram
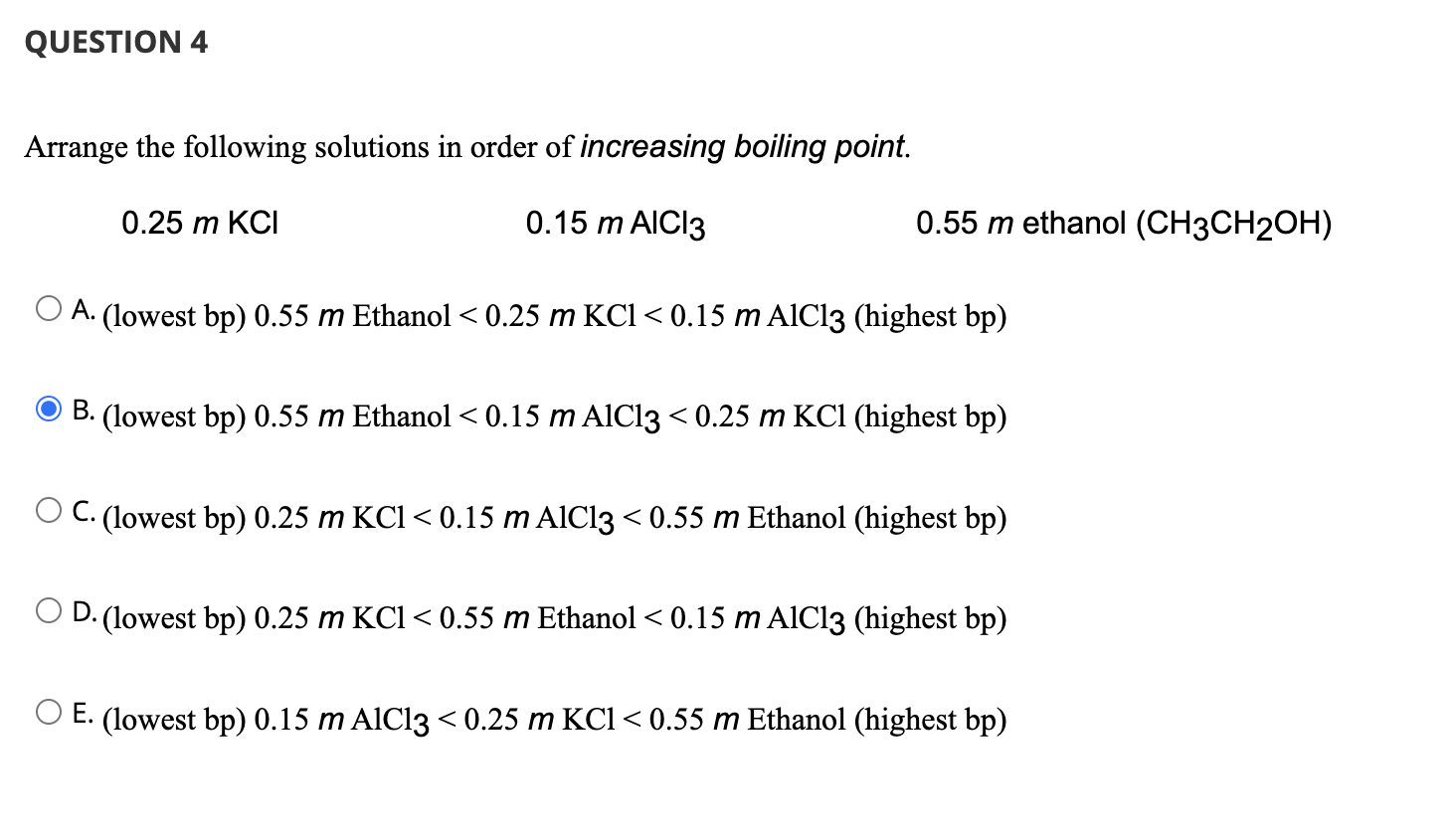
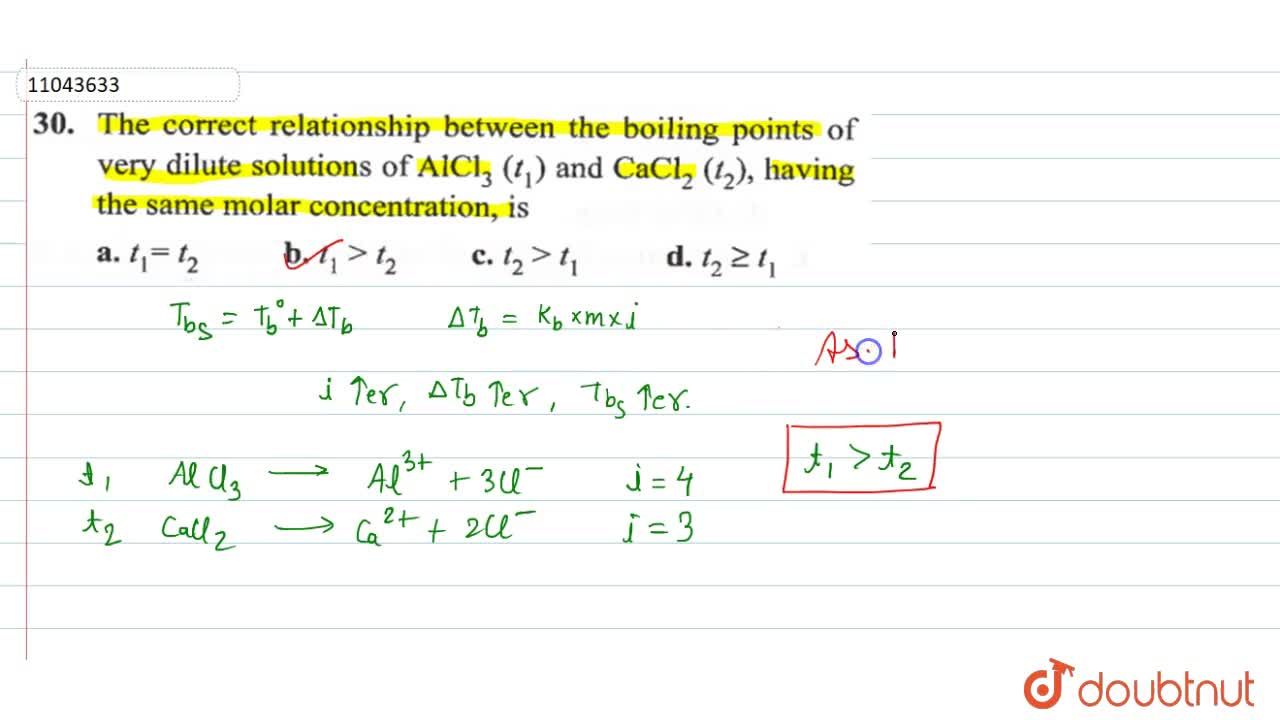
How to arrange the following according to increasing boiling point, freezing point, and vapour pressure: 1m NaCl, 1m CaCl2,1m C6H12O6, and 1m AlCl3 - Quora

Suppose you had a 1.00 m solution of AlCl_3. Assuming complete dissociation, what is the theoretical change in the freezing point of this solution? | Homework.Study.com

Catalytic Dehydration of Carbohydrates Suspended in Organic Solvents Promoted by AlCl3/SiO2 Coated with Choline Chloride - Yang - 2015 - ChemSusChem - Wiley Online Library
Survey of Properties of Key Single and Mixture Halide Salts for Potential Application as High Temperature Heat Transfer Fluids f
Molality Chart Problem Problem Convert Convert Colligative Properties Where it is used Boiling Water